Synthetic polyisoprene has become an important polymer for medical fluid control components.
Bob Ferguson, Vernay Laboratories

(Image courtesy of Vernay Laboratories)
Natural polyisoprene (NR) and synthetic polyisoprene (IR) are rubber thermoset materials with useful properties for medical fluid control components and device assemblies. These material properties include extremely high elongation, high tensile and tear-resistance. They also exhibit excellent resilience, or the ability to quickly return or recover to the original shape after deformation.
What it’s good for
Such properties make IR ideal for medical applications requiring the prevention of fluid leakage around surgical instruments, fluid control valves re-sealing against a seat or interface, and pressure-regulating membranes. Specific uses include:
- Catheter valves and seals.
- Laparoscopic introducer valves (trocars, sheath seals).
- Diagnostic slit septum valves.
- Ventilator flapper disc valves.
- Guidewire seals.
- Non-return pressure regulating diaphragms.
- Needless injection site valves.
All of these applications combine dynamic and static sealing requirements. Resilience is a good example, as it is a dynamic material characteristic.
(Image courtesy of Vernay Industries)
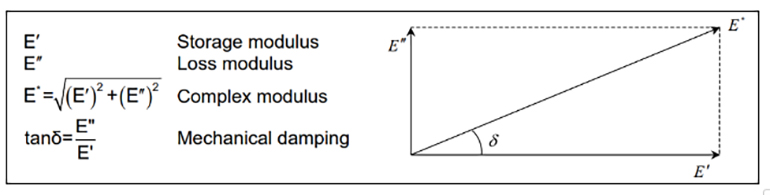
A dynamic mechanical analyzer can quantify dynamic properties and enable comparisons of different rubber recipes in efforts to increase characteristics of resilience. For example, using the DMA allows chemists to quantify the dynamic complex modulus, the storage modulus and the loss modulus.
Years of research have shown that increasing the storage modulus and lowering the loss modulus results in a more resilient recipe with reduced hysteresis.
Think of the storage modulus as the spring component of the complex modulus. In some applications, it is preferred that the rubber part exhibits characteristics like an ideal spring — quick to respond and returning nearly all the energy (not absorbing or dampening energy). The characteristics of the rubber recipe are adjusted using various polymers, fillers, plasticizers and other ingredients, specifically to adjust the ratio of elastic versus loss modulae. This allows chemists to match the resulting rubber material properties specifically to the application and part criteria.
A delicate balance
In addition to the physical characteristics of the IR formulation, meeting specific industry regulations for medical applications is critical. These regulatory aspects include, but are not limited to; biocompatibility (USP Class VI and ISO10993), low-extractable pharmaceutical and in some cases white-listed materials (FDA 21CFR177.2600). These regulations contain strict limitations on performance characteristics and laboratory tests that the materials must meet in areas such as cytotoxicity, hemolysis, mutation, sensitization, limitations on maximum ingredient ratios, restricted ingredients, etc. Translating these requirements into the science and chemistry requires further consideration of effects on material viscosity, rheology and resulting physical properties like modulus, resilience and chemical compatibility. It’s a delicate balance.
Material formulation and properties are important, but they are not the only factors. The elastomeric materials and molded parts depend upon a balance of 3 critical technical aspects: formulation material properties, part/tooling geometry and the molding process. Changes to any one will have impacts on the remaining two.
Although synthetic polyisoprene exhibits many beneficial material properties and characteristics, the part geometry and molding process must be considered and balanced together in order to produce a successful product meeting all of the application criteria.
Bob Ferguson is the VP of global research & development for Vernay Laboratories. His expertise lies in rubber material formulation development, nanoparticles as additives, and magnetic and electro-rheologic materials.
The opinions expressed in this blog post are the author’s only and do not necessarily reflect those of Medical Design and Outsourcing or its employees.




