Esco Medical, the IVF business unit of the Esco Group, has received the U.S Food and Drug Administration 510(k) clearance on the Miri Time-Lapse Embryo Incubator. The Miri TL is a multi-room incubator with a built-in microscope and camera that continuously captures images of embryo developments until the day of transfer without any disturbance. This enables a detailed scoring of the embryos cultured, for better prediction of developmental and implantation potential.
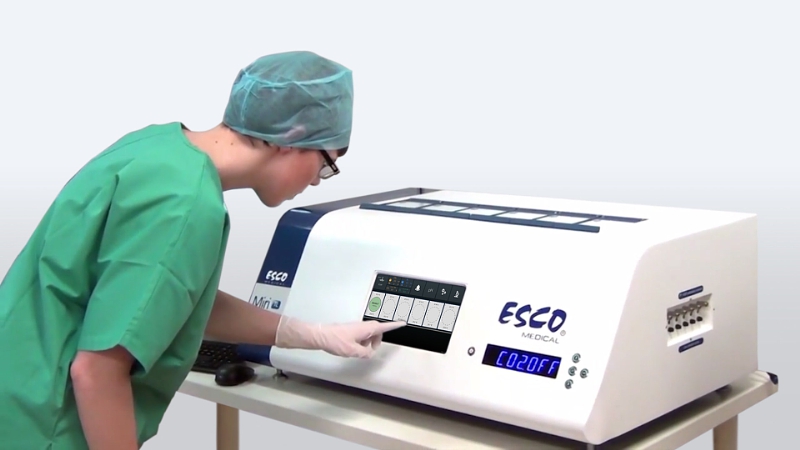
The main screen shows all six chambers as each counter illustrates the duration of time-lapse recording. (Credit: Esco Medical/PR Newswire)
Conventional assessment of embryo selection in IVF is often limited to static observations at pre-defined time intervals, which requires the embryo to be taken out from the incubator. With the Miri TL, the dynamic process of embryo development can be observed without disturbances to culture conditions. This supports the Silent Embryo Hypothesis, which observes that embryos develop better if less disturbed.
The unit has a built-in gas mixer and six individual chambers with independent temperature regulation to ensure optimal embryo culture conditions. It can simultaneously incubate up to 84 embryos in a safe and protected environment. The device includes the Miri TL Viewer Software that assists IVF professionals in processing the data generated.
Esco Medical, a business unit of the Esco Group, is manufacturer of long-term embryo incubators, ART workstations, Anti-Vibration Table, Time-Lapse incubators and other advanced technologies for the IVF industry. Most products are designed in Denmark and manufactured in the EU.




