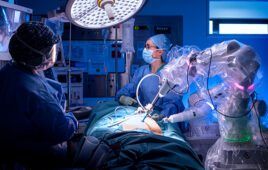
Todd Pope has been working in the surgical robotics space for over a decade. As the CEO of TransEnterix, which has developed Senhance surgical assistance device, Pope believes the surgical robotics market is about to explode.
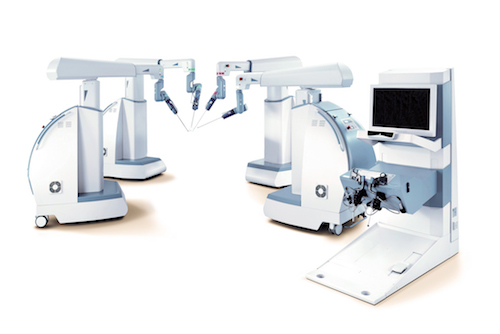 But before it can take off, Pope noted that players have to address a really big problem with robotic surgeries: cost. He also focused on 2 technologies that he believes set Senhance apart, haptics and eye tracking.
But before it can take off, Pope noted that players have to address a really big problem with robotic surgeries: cost. He also focused on 2 technologies that he believes set Senhance apart, haptics and eye tracking.
Senhance is approved for use in the European market and is expected to be introduced to the U.S. market within the next year, under 510(k) clearance. Pope spoke with MDO about what he thinks is important about Senhance and how the company is building its market play.
MDO: One of the biggest criticisms of surgical robotics is cost-per-procedure versus outcomes. How has TransEnterix addressed the issue?
Pope: Let’s face it, there have been thousands of articles written about robotics, and the No. 1 complaint we hear is about cost per surgery. This is from surgeons and hospitals around the world. You have the capital equipment, which is basically the console the surgeon sits at and the arms. Then there are the instruments that plug into the end of the arms, that actually go into the patient and operate (graspers, scissors, cautery, etc.).
In the United States, capital is acquired by a certain budget in the hospital. Hospitals buy capital every year: X-ray machines and C-arms and robots. But when you go down to the operating room, there’s an operating budget as well, and reimbursement codes attached to every action in the OR. When you do a procedure, a hospital is reimbursed. They have to pay for the supplies and pay for the staff, including the surgeon that’s in there.
But per procedure cost is what has been currently driving robotics growth, and the price hasn’t gone down. Right now, the current model uses instruments that can be used for typically 10 cases and then they have to be thrown away.
There’s a reason why there’s only been a very small percent of surgery convert over to robots. It’s not because the robot doesn’t do a good job. The robot does a wonderful job, but it’s just hard to justify increasing your cost.
We have elected to use reusable instruments, and we think that is going to drive more adoptions because it dramatically reduces the cost per procedure. We really believe that we can significantly lower costs using this model and improve adoption rates for the entire market.
MDO: How did you develop the haptics technology? How does it work?
Pope: Developing haptics was a conscious decision –one that took almost 10 years of development. It’s not easy, but there’s some good third party research that confirms the importance of haptic.
When we talked to surgeons, we found there are basically 2 main ways people do surgery today. Either open surgery where there are larger incisions, and the surgeons have their hands inside the abdominal cavity so they can actually feel and touch the organs they’re operating on, or through laparoscopic surgery. Even in laparoscopy, they have their hands-on instrumentation, so they can feel. When they touch an organ or a bone or a vessel, they have different feedback.
We did a lot of work over the years talking to surgeons on what they felt like were the most important senses that they wanted to be maintained or enhanced if they had a robotic interface in between them and their patients. Time and again, by far the number one thing that surgeons said is, “If you can give me haptics, I feel like you’ll give me safety and security,” because anytime you have an interface between the surgeon and the patient, you want to feel like it’s enhancing them.
You certainly don’t want that interface to have anything that it is lacking from their current gold standard. They felt like if they weren’t able to have haptic feedback, they weren’t able to really interrogate tissues, organs, vessels, and be able to feel some of those nuances, that would be an issue. That’s why we focused so much on it. It was just overwhelming feedback from the surgeon community.
The surgeon sits at the console and starts operating. They have their hands in 2 handles, which is very similar to traditional laparoscopic surgery. Our instrumentation works off laparoscopic principles the same way, so when you move your hands up, the tips of the instruments point down. When you move your hands out, the tips of our instruments point in. It’s just like laparoscopic surgery. Then when they have their instrument that they’re controlling, if that instrument were to maybe interact with the stomach versus the pelvic bone, they would feel just like you would think you would feel. The organ has give, and the bone is a hard stop.
We’ve also seen great responses from surgeons when they suture. Surgeons throw a loop, grab the right side and the left and cinch them. We have our testing surgeons close their eyes and they can feel the suture cinch down onto the tissue. This is a delicate process—too tight and you restrict tissue, too loose and you aren’t filling the suture holes.
MDO: You’ve spoken about eye tracking as a market advantage too. How does that work?
Pope: If you think about the current robot technology, the surgeon sits at a console and has their right hand on a handle that controls an instrument that’s on a robotic arm. They have their left hand on another handle that controls another arm of an instrument. Then usually the third arm is the camera. When they need to move the camera (e.g., from the liver to the spleen), they have to stop operating and then engage the camera and move it. That’s can be a real problem when you have to stop and start.
We’ve employed eye-tracking technology to fix that problem. So when the surgeon sits at the console, their right hand controls one arm, their left hand controls another arm. If they need to move, they can press a button and anywhere they look on the screen, their eyes are being tracked. The camera will follow them and change the point of view. If you’re looking at the left lower quadrant on the screen, and you want to look at the right upper quadrant of the abdomen, all you have to do is change the gaze of your eye. The robotic arm that holds the camera will automatically move the camera.
The surgeons love it. It makes them much more efficient. Any time they want to move their instruments to a different part of the body, they just look on the screen, and the robotic arm moves. What we’re enabling them to do with Senhance: they can simultaneously control three robotic arms instead of two. One controlling the camera with their eyes and the other with their right and left hands.
It makes surgery much more efficient, much more elegant when you’re able to do that. You don’t have to stop and start every time you want to change your point of view.
[Want to stay more on top of MDO content? Subscribe to our weekly e-newsletter.]