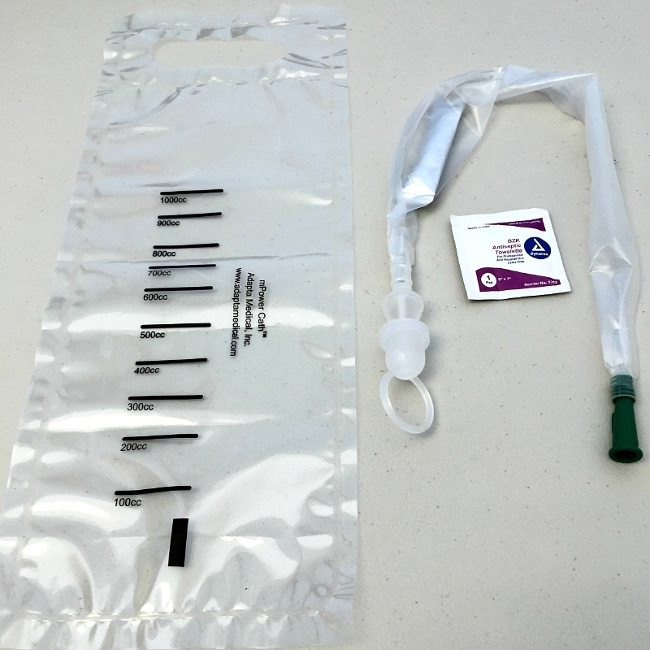
Adapta Medical’s new mPower Hydro-S intermittent urinary catheter system, complete with collection bag. (Credit: PR NewsWire)
Adapta Medical, Inc. has received FDA market release for the PerfIC Cath® intermittent touchless urinary catheter. The sterile catheter system was designed by J. Glen House, MD, a C7 quadriplegic with limited finger dexterity.
FDA clearance for the PerfIC Cath® will result in Adapta expanding the PerfIC Cath® product line and launching the new mPower Cath™ series catheter product line. Both product lines feature hydrophilic and gel lubricants for straight and coude-tipped catheters. The PerfIC Cath® catheters have an attached urine collection bag while the mPower Cath™ products have a urine collection bag that is not attached to the catheter.
“The PerfIC Cath® catheter represents a significant advancement in intermittent catheter technology and is extremely easy to use, even with limited dexterity,” said House. “It is designed for those with a spinal cord injury, multiple sclerosis, diabetes, stroke, spina bifida or other conditions that require a catheter, but can be used easily by those with normal hand dexterity too. It is created for users by users.” With a variety of patents, the catheters are truly unique and offer a new experience for catheter users.
Key features shared by the PerfIC Cath® and mPower Cath™intermittent urinary catheters can be seen at http://adaptamedical.com/amazing-key-features/ and cath411.com include:
- An easy-open package, gripping device and unique sheath that makes self-cathing easy, even with limited dexterity
- A patented gripping device and hydrophilic coating to ensure even and complete catheter lubrication with easy passage into the bladder
- An introducer tip, which bypasses high levels of bacteria to reduce the risk of infection
- A cleaner experience. The catheters are enclosed in a protective sheath that keeps fluid from touching the user, nearly eliminating mess and ensuring easy disposal




