A nasal swab containing a test sample is mixed with a simple lab test that contains a liquid mixed with gold nanoparticles attached to a molecule that binds to the novel coronavirus. If the virus is present, the gold nanoparticles turns the solution a deep blue color (bottom of the tube). If it is not present, the solution retains its original purple color. (Image from University of Maryland School of Medicine)
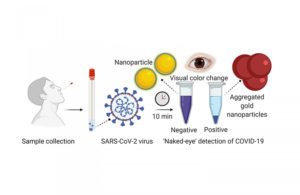
Scientists from the University of Maryland School of Medicine have developed an experimental diagnostic test for COVID-19 that they say can visually detect the presence of the virus in 10 minutes.
It uses a simple assay containing plasmonic gold nanoparticles to detect a color change when the virus is present. The test does not require the use of advanced laboratory techniques, such as those commonly used to amplify DNA, for analysis. The authors published their work last week in the American Chemical Society’s nanotechnology journal ACS Nano.
“Based on our preliminary results, we believe this promising new test may detect RNA material from the virus as early as the first day of infection,” said study leader Dipanjan Pan, a professor of diagnostic radiology and nuclear medicine and pediatrics at the university, in a news release. “Additional studies are needed, however, to confirm whether this is indeed the case.”
RNA is extracted from a nasal swab or saliva sample using a simple process that takes about 10 minutes, according to the researchers. The test uses a highly specific molecule attached to the gold nanoparticles to detect a particular protein that is part of the genetic sequence that is unique to the novel coronavirus. When the biosensor binds to the virus’s gene sequence, the gold nanoparticles respond by turning the liquid reagent from purple to blue, they explained.
“The accuracy of any COVID-19 test is based on being able to reliably detect any virus. This means it does not give a false negative result if the virus actually is present, nor a false positive result if the virus is not present,” Pan said. “Many of the diagnostic tests currently on the market cannot detect the virus until several days after infection. For this reason, they have a significant rate of false negative results.”
Pan has created a company called VitruVian Bio to develop the test for commercial application. He plans to have a pre-submission meeting with the FDA within the next month to discuss requirements for getting an emergency use authorization (EUA) for the test.
Although more clinical studies are warranted, this test could be far less expensive to produce and process than a standard COVID-19 lab test, according to the university. It does not require laboratory equipment or trained personnel to run the test and analyze the results. If this new test meets FDA expectations, it could potentially be used in daycare centers, nursing homes, college campuses, and work places as a surveillance technique to monitor any resurgence of infections, the researchers said.



![A photo of the Medtronic GI Genius ColonPro polyp detection system flagging a potential sign of colon cancer during a colonoscopy. [Photo courtesy of Medtronic]](https://www.medicaldesignandoutsourcing.com/wp-content/uploads/2024/04/Medtronic-GI-Genius-doctors-268x170.jpg)
