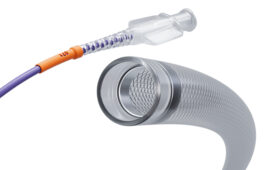
Vascular Graft Solutions has reported the first clinical use of its Frame external support technology in high-flow arteriovenous (AV) fistulas. The first five cases were performed by Dr. Vladimir Matoussevitch from Cologne University Hospital in Germany.
The braided cobalt chrome, kink-resistant external support was designed for autologous vein grafts in bypass or reconstruction of peripheral arterial blood vessels. Mattoussevitch used them to prevent high-flow AV fistula and aneurysmal enlargement complications, which are common in patients undergoing routine autogenous AV fistula access for hemodialysis. The complications have severe clinical and cosmetic impact.
“High-flow AV access increases cardiac output, which may lead to eccentric left ventricular hypertrophy, a key factor in the development of congestive heart failure in hemodialysis patients,” Mattoussevitch said. “In addition, aneurysmal enlargements are at high risk to develop active bleeding, rupture and infection.”
Frame is a CE-marked, kink-resistant, cobalt chromium external support indicated for peripheral arterial bypass and reconstructions surgeries. In AV fistula reconstruction surgery, the aneurysm sac is resected and the vein diameter is reduced to 5-6mm. After downsizing, Frame is applied over the repaired vein to prevent future dilatation and aneurysm recurrence. Limiting the AV fistula outflow with Frame may also prevent the development of high-flow fistula and heart failure, according to the Tel Aviv-based company. The device has the CE Mark.
Mattoussevitch reported that post-procedure flow in the initial five patients was reduced dramatically. Frame has been used in different peripheral reconstruction procedures that required the support of venous segments, according to Dr. Eyal Orion, founder and CEO of Vascular Graft Solutions. “We are looking forward to working together with vascular surgeons to expand our experience with Frame.”