An international clinical trial that studied wearable cardioverter defibrillators (WCDs) found that the devices did not significantly reduce sudden cardiac death – the primary goal of the device – among patients assigned to the device in the first 90 days after a heart attack, but did lower mortality among those who wore it as prescribed, according to a study led by researchers at UC San Francisco.
The devices, which consist of highly sensitive vests that can deliver an electric shock when the heart beats out of rhythm, are designed to provide medical intervention around the clock for patients who have had a heart attack and are at further risk of death due to arrhythmia. The study appears Sept. 27, 2018, in the New England Journal of Medicine (NEJM).
“It remains unclear how to reduce the risk of arrhythmic death definitively, beyond what is possible with appropriate medical therapy, in the early period after heart attack before implantable cardioverter defibrillators are suggested,” said UCSF Health cardiologist Jeffrey Olgin, MD, lead author of the paper and chief of the Division of Cardiology at UCSF. “However, given the totality of the data, the WCD may be reasonable in high-risk patients who are likely to wear the WCD based on shared decision-making.”
Patients who are prone to fast heart rhythms that can cause sudden death typically receive a surgically implanted type of cardiac pacemaker known as a cardioverter defibrillator (ICD). However, current medical guidelines recommend waiting at least 40 days after a heart attack before implanting the device, and 90 days if the patient has had a vascular stent implanted or has undergone bypass surgery.
The guidelines recommend considering a wearable device for a wide range of patients at risk of sudden cardiac death, including those who have low ejection fraction – a measure of the heart’s pumping ability – following a heart attack.
“Previous research has shown the risk of mortality in low ejection fraction patients is highest in the first 90 days after a cardiac event, such as a heart attack or a new diagnosis of heart failure,” said UCSF Health cardiologist Byron Lee, MD, MAS, professor of medicine and the Reeves Endowed Chair in Arrhythmia Research at UCSF and study co-principal investigator.
WCDs aim to bridge this sensitive period before a pacemaker can be implanted.
e appropriate shock during the study, while nine (0.6 percent) received an inappropriate shock. About 70 percent of participants who had an appropriate shock survived to 90 days. Of the 48 participants who died, only 12 were wearing the WCD at time of death.
Mark J. Pletcher, MD, MPH, a UCSF professor of epidemiology and biostatistics who leads the VEST Data Coordinating Center.
The researchers are exploring new analyses for estimating the true causal effect of wearing the WCD, Pletcher said.
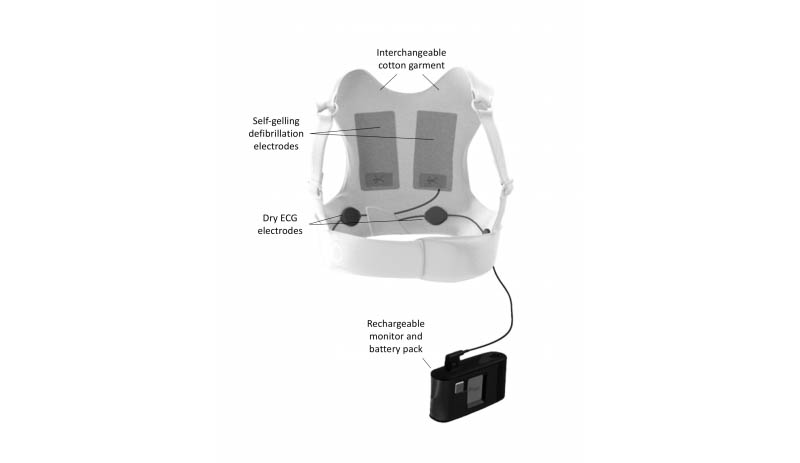
The ZOLL LifeVest wearable cardioverter defibrillator.




