A close-up of one end of the fake wrist. (Credit: National Institute of Standards and Technology)
What if there were a wearable fitness device that could monitor your blood pressure continuously, 24 hours a day?
Unfortunately, blood pressure (BP) measurements currently require the use of a cuff that temporarily stops blood flow. So a wearable BP “watch” using today’s technology would squeeze your wrist every few minutes, making it impracticable to use – not to mention annoying.
A better method might gauge subtle pressure changes at the surface of your skin above one of the main wrist arteries – the radial artery – without regularly cutting off your circulation. But before scientists can create this new technology, they need to understand what the pressure inside a blood vessel “looks” like on the surface of the skin. And to do that, they must make a physical model that can be used to test wearable devices in a laboratory.
National Institute of Standards and Technology (NIST’s) Physical Measurement Laboratory (PML) is currently collaborating with Tufts University’s School of Medicine to develop just such a model, a blood pressure wrist “phantom” – essentially a fake arm that mimics the mechanical properties of blood pulsing through an artery surrounded by human tissue.
“The phantom will give us very precise measurements – say, for example, what is actually the force on the blood vessel wall? And what is the force on the soft tissue and the skin?” says Tufts University School of Medicine assistant professor Mohan Thanikachalam, who is collaborating with NIST on this work. “I think it will help us tremendously in terms of optimizing our technology” for wearable BP devices.
The NIST-Tufts blood pressure phantom consists of a slab of squishy silicone, which stands in for human tissue, sitting on top of a metal plate, the stand-in for bone. A pliable tube runs through the silicone to mimic an artery, through which fluid flows via a mechanical heart pump.
The materials were carefully selected to match the properties of skin, soft tissues, bone, and artery walls, the researchers say. But unlike actual live human tissue, the phantom can easily have sensors running through it, measuring the pressure changes that occur each time water is pumped through the tube.
“One of the things we want to understand is, if a sensor is sitting up here on top of the silicone, what is it really seeing?” says Zeeshan Ahmed, lead researcher for the NIST PML team. “Is it just seeing the primary wave from a pulse of fluid going through? Is it seeing a lot of reflection waves, when the primary wave bounces off the metal plate? How does the pressure change over the time it takes for each pulse of water to pass through the artificial artery?”
The sensors they are currently testing are thin optical fibers called Bragg gratings, designed to block a specific frequency, or color, of light from passing through them. When the pressure changes inside the Bragg grating, so does the color of light that is blocked. Researchers can use this change in color to identify the pressure that was applied to the fiber. The final phantom will likely incorporate about a half dozen of these Bragg sensors, running through the silicone and over its top as well as inside and outside the artificial artery.

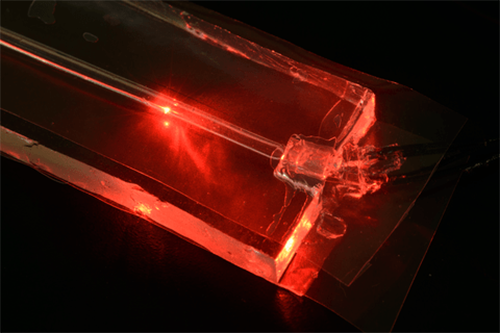
This version of the fake wrist includes the “artery,” sitting in the center of the squishy silicone pad that acts like human skin and soft tissue. Thin fiber optic sensors are embedded in the silicone pad; you can see their extensions coiling around the table at the top of the image. Note: To make it visible in this photograph, the artery is illuminated by a red fiber optic cable that is not part of the actual experiment. (Credit: National Institute of Standards and Technology)
Presently, the NIST team is conducting preliminary tests to gauge the performance of their sensors using a prototype without the mock artery. Instead of pumping water through a tube, they apply pressure to the silicone by crushing it with weights. For example, to mimic a BP of 140/60, they use masses of about 1 to 1.8 kilograms (kg, thousand grams, equivalent to approximately 2 to 4 pounds).
So far, they have found that their sensors are able to detect pressures from 170 millimeters of mercury (mmHg, equivalent to about 22.5 kilopascals, kPa, or about 3¼ pounds per square inch, psi) down to 60 mmHg (about 8 kPa, or a little more than 1 psi) with a resolution of 2 mmHg (about 250 Pa, or less than 0.04 psi). In terms of weight, this means that they are measuring masses of about 1 kg with a resolution of just 20 grams.
Also promising, Ahmed says, is that the results are reproducible: Each time the silicone is crushed, it springs back to its original form, so that the results are the same no matter how many times the experiment is run.
The NIST team, which includes Kevin Douglass, is currently preparing to test the sensors’ ability to measure pressures that change over time, using a universal testing machine that they call “the crushinator.”
If all goes well, the collaboration could potentially have a working prototype sometime this year.




