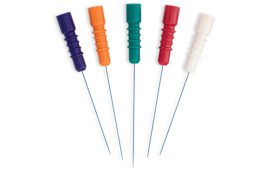
[Image from Florida Anodize System & Technologies]
Aluminum anodizing is the electrochemical passivation process by which the surface layer of an aluminum substrate is converted into an aluminum oxide layer. While a natural oxide layer can be found on aluminum, this layer is often uneven, thin and offers poor protection. The controlled application of an electrical charge in an acidic electrolytic bath results in a very regular and uniform layer that has increased durability, as well as wear and corrosion resistance. Additionally, these anodic layers can undergo secondary processing to incorporate various functional materials such as colorants or lubricants.
There are numerous processes and standards that apply to aluminum anodizing, the most common find their origins from the defense, aerospace and automotive industries. In the U.S., the most often cited anodizing specification is the U.S. Defense specification MIL-A-8625 which defines three types of aluminum anodizing Type I – Chromic acid anodizing, Type II – Sulfuric acid anodizing and Type III – Sulfuric acid hard anodizing, with Type II and Type III being the most often utilized.
How are anodic coatings applied?
The anodizing processes involve submerging the aluminum component into an acid electrolytic bath and then passing an electrical charge through the medium. A cathode is located on the outside of the tank, while the aluminum serves as an anode (hence the term anodizing). As the current moves through the bath, oxygen ions are released from the acid electrolyte and join with the aluminum substrate producing the aluminum oxide layer. It is important to note that, unlike a paint or plating process, the anodic layer is fully integrated into the underlying substrate actually forming into and out of the substrate at the same time.
What you should look for in an anodic coating?
People often misinterpret the terminology of anodizing, especially in regard to the phrase “hardcoat.” While on the face of it, the word hard would seem to indicate some form of strength or wear resistance, in this instance, hardcoat is more accurately referring to the thickness of the anodic layer. The specification from which the term is derived, MIL-A-8625, does not even mention any hardness characteristics for either Type II or Type III anodizes. In fact, the hardness of the aluminum oxide of both types would be equivalent, though the difference in thickness of the hardcoat does significantly alter the surface appearance of the substrate.
This, in turn, leads to what is the ideal anodic coating for medical devices. As stated earlier, the most common anodizing specifications in the U.S. come from the aerospace and defense industries. The needs of these industries are far different than those of medical and surgical devices. For example, the mechanical considerations of the aerospace, defense and automotive industries necessitate abrasion resistance and so a hardcoat anodize would be appropriate. However, for the medical and surgical industries, abrasion resistance is not as high a concern as chemical resiliency as would be needed for sterilization systems.
So, first and foremost, it would be imperative that a medical anodize be durable enough to withstand at least 50 cycles (and preferably more) of ethylene oxide, hydrogen peroxide or high alkaline cleaner. This would require it to have a non-leaching colorant that does not fade, peel or blister after repeat sterilization processing.
Additionally, besides thickness considerations or chemical resiliency, a good medical anodize should also have a smooth and even finish with no localized discoloration, and colors should not appear dull or muddled (unless that is the desired appearance). Rather, for easy identification and human factors considerations in the medical setting, the anodic coatings should have a vibrant, easily identifiable and lustrous finish.
Finally, as human factors assessments in medical device design are becoming ever more stringent, a medical anodize should be available in a multitude of colors to assist operators in making an easy distinction between device types or control surfaces. While black or clear finishes are available, a palette of non-leachable, sterilization resistant colors enables medical device manufacturers to effectively utilize color as a human factors modality.
In summary, a medical-grade anodize should be:
- an even and consistent finish,
- be available in a multitude of non-leaching colors,
- be aesthetically pleasing,
- resistant to the harsh chemical environments of sterilization systems
What to look for in an anodizing provider
Anodizing providers are located in almost every corner of the United States. However, in the medical device industry, the cost of non-quality is exceptionally severe, and so manufacturers must be vigilant in their selection of an anodizing provider.
When selecting a vendor, a few important things to consider include:
- Medical industry experience – most anodizing is performed for the aerospace and defense industries, so selecting an anodizing company not just experienced, but with specialization in medical devices can drastically impact the finished quality of the anodic coating. Acceptable machining oils, manufacturing standards, etc. are all industry-specific, so processing medical equipment in chemical baths used for other industries can lead to bath contamination and nonconforming material.
- Medical industry compliance – when all components are critical-to-quality, it is important that a vendor understands the regulatory requirements of the medical device sector and have an industry-specific audited Quality Management System such as ISO 13485.
- Validated and scalable production – The consistency and quality of the finished anodic coating must be measured and validated at a level acceptable for scalable production. When selecting a vendor, it is appropriate to ask about a company’s QC pass percentage, particularly as in the medical device industry the costs associated with noncomplying/nonconforming materials returns can be both financially significant, and also a regulatory complication. A QC pass rate of 98% or greater is usually a signal of a high-quality medical-grade anodic process.
Neel Patel is a VP at Florida Anodize System & Technologies (FAST) in Sanford, Fla. For more information, please visit anodizefast.com or email sales@anodizefast.com.
The opinions expressed in this blog post are the authors’ only and do not necessarily reflect those of Medical Design and Outsourcing or its employees.