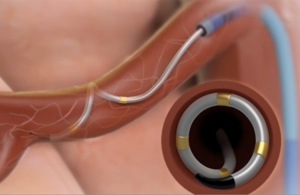
The Symplicity Spyral device delivers energy to the nerves in the wall of the artery leading to the kidney. [Image courtesy of Medtronic]
The hope now is to win FDA approval in 2023. (Update: Medtronic’s RDN system won FDA approval in November 2023. You can read more about Medtronic’s lessons learned, what’s next for the technology, and other potential applications.)
To better understand the technology behind what Medtronic leaders expect to become a multibillion-dollar business, Medical Design & Outsourcing spoke with Jason Weidman, Medtronic SVP and president of the coronary and renal denervation business, which is part of Medtronic’s cardiovascular portfolio. (We’ve edited this conversation for brevity and clarity.)
MDO: What’s the simplest way to explain renal denervation?
Weidman: The basic idea is that your kidneys are part of the body’s blood pressure control mechanism and communication happens to and from the brain and the kidney on how to regulate that kidney’s activity. That communication travels through nerves that are inside the walls of the artery that leads to the kidney. Patients with high blood pressure, though, often have overactivity in these nerves, so that communication isn’t working right. If we can interrupt that overactivity, we can bring down blood pressure.
Renal denervation is a minimally invasive outpatient procedure, and in many respects, it’s quite similar to a coronary stenting procedure, except there’s no permanent implant. It’s that level of invasiveness, that level of ease of procedure.
Through a tiny incision in your leg, a small device is inserted up through the vessels to that artery that leads to the kidney. Then our device, the Medtronic Symplicity Spyral device, delivers energy to the wall of that artery where those nerves are running through, with the purpose of basically calming that overactivity. This is, in essence, what can help to lower blood pressure.
MDO: What’s special about Medtronic’s Symplicity Spyral device design?
Weidman: When it was first dreamt up a decade ago … there was a design of a device that was just a single point that you would have to rotate around and try to touch it and apply this energy several different times in the vessel.

Medtronic’s Symplicity Spyral device is a multi-electrode catheter for renal denervation. [Image courtesy of Medtronic]
MDO: How did that design come to be?
Weidman: It was just our engineers trying to come up with a better way to do the procedure and to take variation out of it.
MDO: What kind of energy are you using?
Weidman: There are other people that are trying to do renal denervation, and there are different modes of energy that can be used. We’re using RF energy, or radiofrequency energy. That’s the other unique aspect. Some competition is using ultrasound. Other people are trying to use direct injection of chemical agents that could interrupt those nerves.
MDO: What’s the advantage of RF?
Weidman: One of the reasons why we like RF is, as we viewed this from the beginning, we’ve done a lot of clinical work, and we’re very comfortable with how that energy then disperses into the vessel and impacts the nerves. And through all of the work that’s done, we are able to selectively interrupt that activity of the nerves like we intend without really doing any other damage to the vessel that you wouldn’t want to have. The other advantage of our RF system is that when you put that all into a catheter, it makes for an overall design that is incredibly easy for the physician to maneuver and to get to where it needs to go for this procedure, regardless of, for instance, how tortuous the anatomy is. It’s a system that’s very similar to what they would use in a coronary stent system, so it’s familiar to the physician that will do this type of procedure.
MDO: Are the components made entirely by Medtronic, or are there significant pieces that come from contract manufacturers or other suppliers?
Weidman: The final assembly and the final manufacturing of the devices is done by Medtronic. Like almost every other Medtronic product, we have countless suppliers and sub-suppliers of different components. We’re not 100% vertically integrated.
MDO: Why would a patient choose RDN for hypertension?

Jason Weidman is a Medtronic SVP and President of the Coronary & Renal Denervation business. [Photo courtesy of Medtronic]
The two most common ways to treat hypertension today are lifestyle changes and drugs. And particularly when we look at pharmaceuticals, we know that they generally work in most patients, but the issue is people just don’t take them. A lot of studies out there look at compliance, or more specifically, noncompliance with the blood pressure-lowering medication. And at a year, what you’ll see is only about 50% of patients are partially compliant to their meds. So only half the people are fully taking their meds. Then you’ve got one-fifth of people that aren’t taking them at all. Even though the drugs work, people aren’t really taking them like they should be, and that’s why you have so few people at their target blood pressure.
RDN provides this other option, and essentially you don’t have to worry about compliance. It’s a one-time procedure, there’s no implant, you go in, you do it once, and it’s always on … not something that is dependent on you constantly remembering to take the pill. Patients are really interested in having an option of a procedure to lower blood pressure.
MDO: Why would someone not want to undergo renal denervation?
Weidman: If someone is uncomfortable having a procedure, that’s why they wouldn’t want to go down the route. But I would say even though the trials are ongoing, we do have several trials completed and thousands of patient experiences in our clinical trials that are completed. The complications are less than 1%.
MDO: Do you have updated timing on when you hope to secure approval?
Weidman: We have four randomized, sham-controlled studies, three of which are complete and successful, showing safety and efficacy. The last one of those studies is called the HTN-ON MED study, and we expect to have the data for that study available in the second half of next year. And then, we would submit for approval in the U.S. and China shortly thereafter. At that point, you’re kind of in the hands of the regulatory bodies, but for a brand-new-to-world therapy, FDA approval comes somewhere around a year after. I would hope it would be by the end of 2023.
This story was originally published in November 2021 and updated in December 2023.




