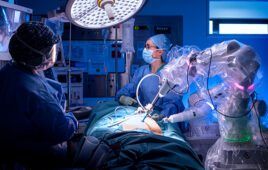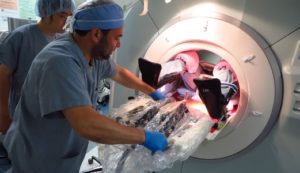
Physicians at Brigham and Women’s Hospital perform a biopsy using WPI’s robot. [Image from Worcester Polytechnic Institute]
PracticePoint, with $17 million in initial funding, will focus on creating smart and secure medical devices that can interact with the physical world and improve patient-centric care.
“Technology is changing medicine, and PracticePoint is designed to help ensure our Commonwealth stays on the cutting edge of this important field to advance economic prosperity, but, more important, to advance health in our communities,” said WPI president Laurie Leshin, in a press release. “When we bring together creative engineers, scientists, clinicians, companies and entrepreneurs to work together in a novel setting like PracticePoint, I believe the results will be extraordinary.”
The PracticePoint program received a $5 million capital grant from the Massachusetts Technology Collaborative to help build the facility that will be located at Gateway Park. GE Life Sciences matched with a $2.5 million capital and imaging equipment grant. WPI has also contributed $9.5 million for facility, faculty and staff and operational support. UMass Medical School and MITRE, WPI partners, have also pledged faculty or staff time and research support.
This new innovation center is a membership-based research, development and commercialization alliance that is designed to create innovative technologies and launch medical cyber-physical systems. The facility will mimic point-of-practice environments that are associated with advanced manufacturing and testing equipment.
Teams will be able to work together under one roof to get smart medical devices from prototype to product quicker and more effectively.
“We will take the technology and innovation we develop here and scale it across the world to solve some of the toughest challenges we face,” Leshin said.
WPI has done significant research in robotics, sensors, data science, cybersecurity and systems engineering. The launch of PracticePoint allows each stage of product development to be reached.
“Developing medical devices is challenging,” said Greg Fischer, an associate professor of mechanical, biomedical and robotics engineering and the director of PracticePoint. “This is particularly difficult when you start working with smart medical devices.”
Smart and secure medical devices are important for patient protection. Some devices can monitor vitals, diet, hydration and send that data to clinicians. Other smart devices are surgical robotic systems or other assistive devices that help surgeons perform minimally-invasive surgeries with precision.
“By putting a manufacturing facility side-by-side with a clinical environment, we can go back and forth between development in a machine shop or an electronics lab, develop the technology, test it, evaluate it and bring it to the clinical setting,” Fischer said. “We can significantly accelerate the timeline of launching a product.”
The design of the facility is going to mimic facilities that officer patient-centered care. It will include four testing suites that have a surgical/imaging area, a controlled-care setting (like an emergency room or ICU), rehabilitation/assistive care center and a residential setting that will act as a private home or nursing home. The equipment in the PracticePoint facility will test for cyber vulnerabilities and provide big data collections for relevant populations.
[Want to stay more on top of MDO content? Subscribe to our weekly e-newsletter.]