Siemens Healthineers has announced a FDA 510 (K) clearance for Xprecia Stride Coagulation Analyzer, a hand-held portable analyzer used for professional point-of-care use. This device is able to measure Prothrombin Time International Normalized Ratio– how quickly blood is clotting for people taking blood thinners. According to Siemens Healthineers, these tests are often conducted on those who experience atrial fibrillation, heart valve replacement surgery, deep vein thrombosis and congenital heart defects.
(Credit: Williams Medical)
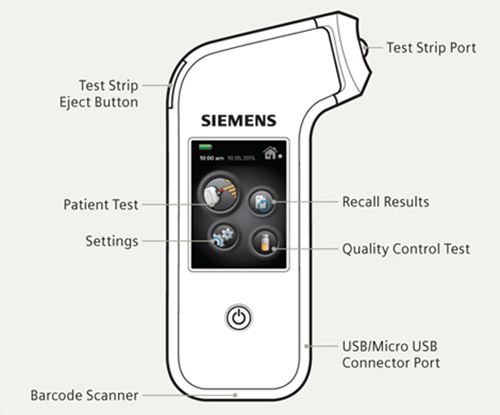
The 1.6-inch-by-6.7-inch analyzer weighs 10.5 oz, about the size of a cellular device and can be used at any angle. The device just needs to directly touch the patient’s finger for a blood sample to be taken. Results and delivered in minutes.
For easy use, the Xprecia Stride analyzer includes a barcode scanner to make data collection and retrieval simple and uses icons and a colorful display, similar to a smartphone, that is not usually installed in medical devices. The device also includes a test-strip injection button that allows the strip to be discarded within touching the strip itself, allowing for safer use.
Extra features include three AA batteries, four interchangeable colored caps and a USB cable used to transfer data and to update the device’s system. The material used to make the Xprecia Stride designed to last for years in hospital conditions without breaking and can withstand being cleansed frequently with chemicals.




