
Ethylene oxide
The first six of what attorneys said could be more than 100 lawsuits were filed recently in Georgia against Sterigenics and Becton Dickinson (NYSE:BDX) by people who have lived, worked or attended school near those companies’ medical device sterilization plants.
The plants use ethylene oxide (EtO) to sterilize millions of devices per year and reportedly have emitted thousands pounds of the gas into the atmosphere over the past. The U.S. Environmental Protection Agency named EtO a Level 1 carcinogen in 2016. Two years later, the EPA’s National Air Toxics Assessment identified 12 areas of the country with elevated levels of EtO emissions and associated cancer risk, including the areas of Georgia where BD and Sterigenics have EtO plants.
The plaintiffs in the Georgia cases have been diagnosed with leukemia, lymphoma or breast cancer, according to attorney Cale Conley of Conley Griggs Partin in Atlanta. Conley said he has filed personal injury lawsuits against Sterigenics, its parent company, Sotera Health, and others.
“These are just the first cases that have been filed,” Conley said in a press conference on Tuesday. “We expect over the coming weeks and months there will be many others.”
“Sterigenics empathizes with anyone battling cancer, but our Atlanta facility’s safe operations are not responsible for causing the illnesses alleged in these lawsuits,” said company spokesman Bryan Locke. “We intend to vigorously defend against the plaintiffs’ unfounded claims.”
“We anticipate that a tremendous amount of information will be learned during the discovery of these cases,” said Darren Penn of Atlanta-based Penn Law, lead attorney on the cases against BD. “It is our intention to get that information out to the public.”
The attorneys described some plaintiffs’ illnesses, including that of Gena McLendon, 68, who worked at healthcare facilities within 3 miles of the BD plant for 25 years. McLendon was diagnosed with multiple myeloma in July 2019, is undergoing chemotherapy and radiation treatments, and has had surgery and a bone marrow transplant, according to Penn. “(She) is fighting every single day to do the very best that she can do to survive,” he said.
Pediatrician Lisa Miller, 58, has an office 1 mile from the BD plant and lives 3.5 miles away, according to Penn. She was diagnosed in July 2020 with metastatic ductal breast cancer, has had chemotherapy and radiation and is planning on having a double mastectomy, he said, adding, “She’s in the throes of her treatment.”
Penn also said that Black residents of two neighborhoods located on either side of the BD plant have been “dramatically harmed.”
BD does not comment on pending litigation, but spokesman Troy Kirkpatrick said in an email that “there are vast amounts of air-monitoring data that provide insights to actual levels of ethylene oxide (EtO) present in greater Atlanta and across Georgia.”
The Georgia Environmental Protection Division (EPD) released EtO air monitoring data on August 20, 2020 for a variety of areas around the state.
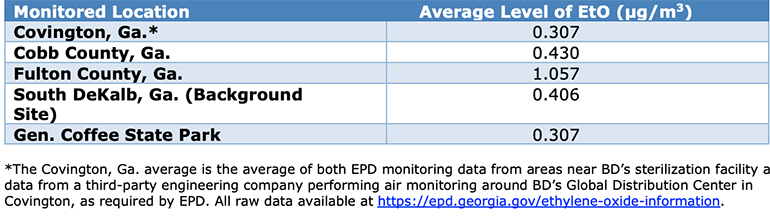
(Chart courtesy of BD)
“This data shows that average levels of EtO in Covington, Ga. are exactly the same as the levels found in the rural General Coffee State Park and below levels found at EPD’s background monitoring station in South DeKalb, Ga. approximately 30 miles from BD’s facility in Covington,” Kirkpatrick said. “The data also show that average levels across the greater Atlanta area are about the same in areas where there are EtO sterilization facilities and areas where there are not EtO sterilization facilities. The data suggests that those who live in communities with sterilization facilities are exposed to similar amounts of EtO as those who do not live near the facilities because of other sources of EtO, including naturally occurring sources.”


