A-1 Engineering produces high tech, high quality molds and tooling. The company primarily serves the aerospace, industrial equipment, medical, power generation, food processing, and optical industries. It specializes in engineering hot runner molds, pneumatic valve gate molds, stack molds, optical lens molds, and compact disc molds, in addition to most types of conventional molds.
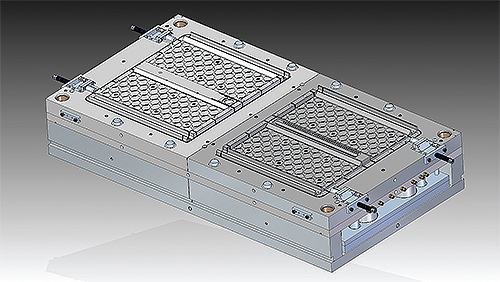
A-1 Engineering used Solid Edge’s new synchronous technology to design this baby-gate mold.
In order to increase their advantage, A-1 owners Dennis Richner and Todd Craft determined that the company could better serve its customer needs by using a mid-range CAD system instead of its high-end and hard to use software. They wanted a replacement system that was easier to learn and use and could accept multiple forms of customer data. During the evaluation process, a member of the A-1 engineering staff attended a Siemens Solid Edge Synchronous Technology (ST) demonstration and recommended further investigation of the CAD product.
He recommended the implementation of Solid Edge’s software. “We receive files from almost every CAD system. Prior to synchronous technology, manipulating the data was virtually impossible but since installing Solid Edge, it’s simple. Moreover, synchronous technology allows us to rebuild only those elements of the model that are necessary to effect the change.”
Work requests for molds and customer data conversion funnel through a team that supports the shop floor staff with the design data they need to optimize five CNC mills, three CNC lathes, several grinders, and tool-making equipment. The data must be accurate because when the product nears inspection or assembly, there is no time for reworking the design. Team members often rely on obtaining a quick dimension or feature relationship for a customer’s design file. The CAD system must effectively support this process.
Synchronous technology is a history-free, feature-based modeling technology that can facilitate a faster design experience. “Synchronous technology represents a breakthrough capability,” added the engineer. “This is what led management to drop its purchase decision of another popular CAD system and implement Solid Edge.”
Dr. Ken Versprille, PLM Research Director at Collaborative Product Development Associates, said, “Synchronous technology from Siemens PLM Software is an innovation in 3D geometry modeling. The technology surpasses traditional parametric, history-based modeling. ST has the speed and flexibility of direct modeling and the precise control of dimension-driven design. The intelligence embedded in ST provides users with the flexibility to work with data from other CAD systems that leading firms require.”
Others at A-1 added “We can edit eyeglass mold ejector pins just by clicking on the ends and changing the length. With traditional history-based CAD, we needed to redraw them. That’s five seconds versus ten minutes times twenty-four pins per mold or two minutes versus four hours.”
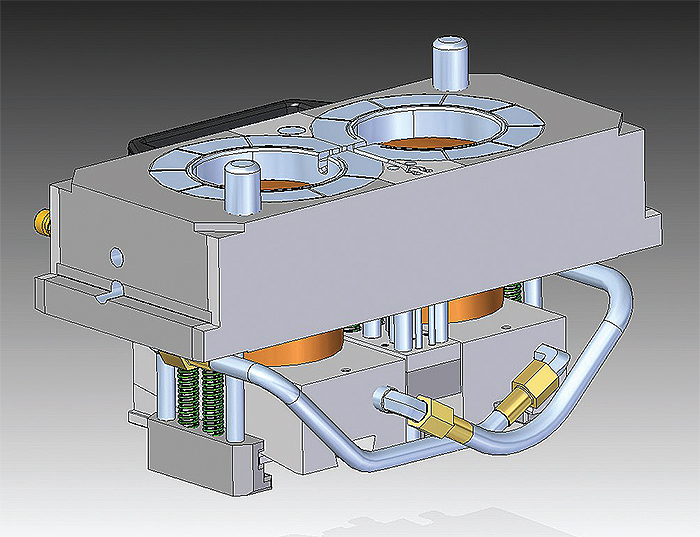
For this eyeglass mold, A-1 engineers edited the mold ejector pins by clicking on the ends and changing the length.
While A-1 Engineering engineers sometimes still need to recreate parts submitted by their customers, the staff says that Solid Edge’s capabilities enable them to sketch parts quickly. In addition, they noted that with some customers, the parts need to be received in wireframe mode. They noted that using Solid Edge, migration from wireframe to 3D is easy.
“Solid Edge is twice as easy to learn as a traditional CAD system,” one engineer stated. “The user interface and design approach are quite intuitive. Overall, it takes only one-third of the time typically needed to change a part. We can take on more business because we can turn designs around faster.”
Siemens PLM Software
www.siemens.com
::Design World::




