Avation Medical co-founders Jill Schiaparelli and Manish Vaishya discuss the design of their wearable Vivally neuromodulation system for bladder control.
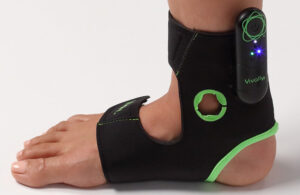
Avation Medical’s Vivally neuromodulation device for overactive bladder patients [Photo courtesy of Avation Medical]
So Avation Medical developed a way to bring neuromodulation to overactive bladder (OAB) patients where they live. Avation’s Vivally System for treating urge urinary incontinence (UUI) and urinary urgency caused by OAB syndrome is the only FDA-cleared, closed-loop, noninvasive neuromodulation system for bladder control.
“Neuromodulation works really well. It is the best clinical treatment we have for OAB and yet only a fraction of a percentage of people are getting it,” Avation Medical co-founder and CEO Jill Schiaparelli said in an interview. “We saw that problem and we said, ‘How do we solve it?'”
The Vivally device delivers electrical stimulation to a patient’s tibial nerve, the same target as Medtronic’s Nuro system for percutaneous tibial neuromodulation. But instead of a weekly needle procedure at a clinic, the Vivally device is worn on a patient’s ankle for autonomously adjusted therapy at home.
How Avation’s Vivally System helps bladder control

Avation Medical co-founder and Chief Technology Officer Manish Vaishya [Photo courtesy of Avation Medical]
The garments come in two adjustable sizes and can be worn on the left or right ankle. The devices are designed to place the stimulation-delivering electrodes reliably near the tibial nerve site of delivery.
The system uses a closed-loop design to confirm activation of the tibial nerve, continuously adjust the therapy during 30-minute sessions once or thrice per week, and accommodate movement and variable factors like humidity, temperature skin condition and BMI.
“We are constantly monitoring the impact of the stimulation on the patient using a muscular response called electromyography (EMG),” Avation co-founder and Chief Technology Officer Manish Vaishya said. “We have found an excellent correlation and we believe EMG is a very, very good indicator of how well you are stimulating the nerve.”
The system monitors EMG in real time and adjusts the stimulation current for therapy uniformity and safety.
The electrodes each measure 22 mm by 50 mm, for an approximate surface area of 996 mm². Vivally says they’re shaped to ensure tibal nerve recruitment and sized for comfortable current density. The electrodes were specifically designed to be tolerant of a device that may be placed slightly out of position.
The system delivers a constant current, rather than voltage control like you’d get from most off-the-shelf systems, Vaishya said.
“If you provide certain voltage, as anything changes — your impedance changes, your skin condition, your electrodes wear out — you’re going to see a significant decline in the current and there’s no way to know,” he said. “Current is really what treats people. So by applying a constant current at all times, we make sure that the system is insensitive to any changing conditions.”
Vivally’s silver ink electrodes are printed using a proprietary printing process developed with a supplier. The electrodes deliver a fixed current of 20 mA. While patch-type electrodes are available off the shelf, those are mostly for sensing currents in microamps.
“We had to really develop the whole process of how to print this stimulation electrode on a variable garment — which is a very specific material, very soft, very breathable, very comfortable — and there are multiple layers of electrodes,” Vaishya said.
The device has seven layers of material — including conductive layers and insulation layers — which helps for comfort and to prevent current leaks into the skin.
The team considered neoprene for the garment, but decided it wouldn’t be durable, elastic, comfortable or breathable enough. Instead, they went with a special material based on nylon. Vaishya declined to offer more detail other than saying it’s a multilayer, proprietary material.
A key component is the gel cushion between the electrodes and a patient’s skin. That protects against high current peaks in patients with flaky skin, and also acts as a DC power block. These gel cushions are reusable for up to a month, making the Vivally devices easier to use than they would be with single-use cushions.
“You just take the garment off, put it back and the gel pad stays with a garment,” he said.
Another custom feature is found in the circuit board, Vaishya said.
“Most off-the-shelf, cheaper versions have monophasic waveforms and we have a perfectly balanced biphasic waveform,” he said. “Whatever charge we put in the positive direction in the body, we put the same amount in the negative with a certain phase lag so that way there’s no charge buildup in the body, while at the same time therapy is being delivered effectively. And that is a huge benefit, I believe, in terms of both comfort and compliance.”
What’s next for Avation Medical

Avation Medical co-founder and CEO Jill Schiaparelli [Photo courtesy of Avation Medical]
Schiaparelli sees a huge opportunity to fill gaps in the collective knowledge about neuromodulation. For example, when Avation launched Vivally, alternatives included implants that delivery 24/7 stimulation and percutaneous stimulation for 30 minutes once per week, therapy dictated by what insurers will cover.
“Nobody has optimized it,” she said. “We have an IRB-approved real world registry and we’ll be able to phenotype a lot of that. We might also be able to say somebody with this symptom profile within this stimulation dosage has the best outcome. Because we’ll have tens of thousands of people in the data. No one has any data like that.”
And while Avation is focused on getting the Vivally System to market, Vaishya anticipates much more to come.
“I do believe that this is a truly platform technology,” Vaishya said. “I know that’s a cliche people use all the time. But the fact that it’s a wearable device [with] closed-loop control I think makes it adaptable to a large number of applications.”