By Jay Tourigny, Vice President of Operations, MicroCare Medical, New Britain, Conn.
Some medical devices are more difficult to clean and coat than others. These tips will help streamline these processes and ensure quality parts and performance.
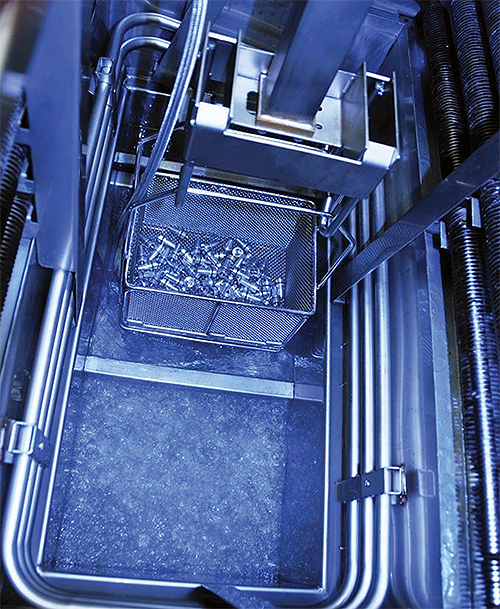
A solvent-based cleaning system such as this vapor degreaser can be an effective part of the cleaning process for devices with small crevices or blind holes.
The right cleaning and coating solution will ensure the best and most consistent performance from a medical device, ensuring that it is clean, sterile, and ready for end users. Most manufactured devices will require some measure of cleaning to remove unwanted contamination that results from manufacturing processes. The trick is to choose a cleaning process that will effectively clean a variety of materials and geometries such as plastic injection molded parts, stainless steel micro tubing, or implantable devices.
Any device which slides, moves side to side, or rotates may be a candidate for a lubricant coating. Devices such as a syringe needle (canoula) for injecting medicine or fluids may be cleaned and then coated with silicone to reduce friction when the needle pierces the skin. Likewise, mechanical assemblies consisting of multiple component parts, such as a surgical stapler, often need a lubricant coating to reduce friction and address stacked tolerances.
Typically, the largest consideration in the cleaning process is cost effectiveness expressed as cost per part cleaned. Another issue is materials compatibility. Other factors include ease of use, safety, and environmental concerns.
Unique surfaces
Devices with small crevices or blind holes can be hard to clean effectively if using aqueous-based cleaning systems. The high surface tension of water makes it difficult for the water to easily flow in and out of confined spaces, often compromising cleanliness. One solution is to use solvent-based cleaning systems because their low viscosity and surface tension ratings allow them to clean very effectively, even in small crevices and areas where water cannot easily flow.
Another challenge is the application of a uniform and consistent lubricant coating on a specific surface while keeping it off adjacent surfaces. This situation is of particular importance for medical devices that require a pristine appearance. Some coatings have the potential to rub off on other parts of the device or the device’s packaging. Advanced lubricants such as polytetrafluoroethylene (PTFE) based dry-lubricants are non-migrating and will not transfer.
Cosmetics matter
By careful selection of a cleaning process it is possible to easily eliminate cosmetic defects such as fingerprints, oils, or particulates as a result of the manufacturing.
Coating a device with a silicone lubricant can leave an oily finish if done incorrectly. Proper mixing and ratio calibration of silicone oil to carrier fluid is necessary to ensure ideal performance and cosmetics. The same is important with dry lubricant PTFE coatings — the ratio calibration of dry lubricant particles to carrier fluid is necessary to ensure the finished coating dries completely and uniformly after application.

This partially assembled surgical stapling medical device has metal and plastic rails as well as a spring which slide against each other. This device has been treated
with the Dura-Glide coating to prevent the stacking of tolerances.
Eliminating bioburden
Medical device end users need to be able to remove bioburden – bacteria and remains from dead bacteria. Since water is a growth medium for bacteria, aqueous-based cleaning systems must be carefully monitored to ensure they do not become breeding grounds for bacteria and associated material.
The use of solvent-based cleaning systems can eliminate bioburden. Solvents create a hostile environment to bacteria growth. If bioburden is not addressed properly, it can lead to infection when the device is used on a patient. Specify a solvent based process with sub-micron filtration to eliminate the possibility of problems in the field.

This stainless steel test coupon is being dipped in a bath of PTFE dry lubricant. This is a qualification test to confirm that micro dispersion is suitable
for use on a device. The test is performed to confirm that the coating is uniform.
Addressing stacked tolerances
The multiple parts of complex assemblies can bind and create user challenges for device actuation. This situation is particularly common when dealing with single-use mechanical assemblies such as surgical staplers and arthroscopic devices.
Designing tighter tolerances into device components is one way to ensure higher levels of precision. However, higher precision products typically are more expensive. The application of PTFE or silicone on the finished assembly can reduce friction caused by stacked tolerances.
Dry lubricants using PTFE particles can reduce the force needed to actuate the device by 25 to 30%. Furthermore, dry lubricants are clean and non-migrating. In fact, many single-use medical devices used today would not be commercially viable without a PTFE coating. Dry lubricants are used on many devices or mechanical assemblies found in the operating room including catheters, cutting tools, staplers, hypotubes, and other surface-to-surface complex assemblies.
Discuss this on The Engineering Exchange:
MicroCare Medical
www.microcaremedical.com
::Design World::




