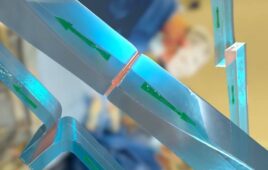
[Image courtesy of Dymax]
The new adhesive has extremely low water absorption of 0.5% and is designed to be autoclave resistant for more than 100 cycles, the Torrington, Connecticut-based manufacturer said in a news release. The material can also protect sensors, RFID chips and other electronic components as an encapsulant.
The material cures quickly when exposed to broad-spectrum UV light and is optimized to be LED curable at a wavelength of 365 nm, Dymax said. The 1040-M adhesive bonds to stainless steel, aluminum, glass, PP/PE, PCB and other substrates, and can be used for medical devices such as scopes, vials, tools and dental equipment.
Dymax also said that 1040-M meets the requirements for ISO 10993-5 cytotoxicity, is formulated without IBOA, has a durometer hardness of D60 and viscosity of 775 cP.
The product is solvent-free and RoHS-compliant, “making it a ‘greener’ choice over comparable one- and two-part solvent-based epoxies currently on the market,” the company said.