(Image courtesy of Origami Surgical)
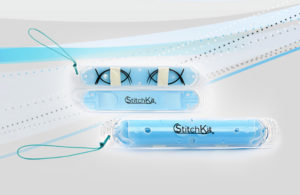
Origami Surgical announced that the FDA has cleared StitchKit PARK, a device that enables surgeons to customize the contents of its StitchKit suture-delivery device with the sutures of their choice.
The StitchKit PARK cartridge allows the surgeon to pre-load the desired sutures for any procedure. Once loaded with needles and sutures, the device is closed and delivered into the surgical field through a trocar, according to the Madison, N.J.-based company. Once inside the abdomen, the device is opened, and the surgeon pulls out the first needle to begin suturing.
When suturing is complete, the needle is cut off and placed into the disposal compartment, which has two “trap doors” securing the needles inside the device. The disposal compartment is transparent so the needles may be counted before the device is removed, to help ensure that no needles are left behind inside the patient. When all suturing is completed, the device is closed, and a retrieval string is provided for easy grasping and removal, the company said in a press release.
Origami Surgical said it developed technology to facilitate minimally invasive robotic procedures with time-saving outcomes.
“StitchKit is a significant benefit as it allows us to perform robotic procedures more quickly and efficiently,” said Dr. Kimberly Kenton, chief of female pelvic medicine and reconstructive surgery in the Department of Obstetrics and Gynecology at Northwestern Medicine in Chicago.




![A photo of the Medtronic GI Genius ColonPro polyp detection system flagging a potential sign of colon cancer during a colonoscopy. [Photo courtesy of Medtronic]](https://www.medicaldesignandoutsourcing.com/wp-content/uploads/2024/04/Medtronic-GI-Genius-doctors-268x170.jpg)