Two experts explain how design engineers can get the most valuable information out of clinical trials.
Kris Godbey and Robin Huneke Rosenberg, 3M
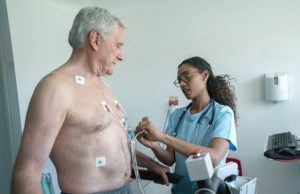
(Image from Getty Images)
You finally have reached the time where you get to evaluate your product in a clinical trial. There are many important steps to keep in mind when advancing your project to this next level. Here are seven that you should not skip:
1. Call a lawyer
The clinical trial is just one of the many steps in an iterative product process. Before sharing information on your product with external parties, work with an attorney to help with guidance on documentation needed for clinical trials. This step is often overlooked but is a critical component that can help protect your proprietary information.
2. Do your homework
Clinical trials are carried out to determine whether your product will perform as intended. Planning for a clinical trial is a process that may require your team to assist with the planning and design of the clinical protocol. As the product engineer, you may be providing input to the clinical trial design, submitting product materials and supporting reports to verify that the product is safe for human use.
As you begin to design and plan a clinical trial, consider how other companies with similar products approached their clinical trials. Consult public information sites as Clinicaltrials.gov to review information about clinical studies with similar products. For medical clinical trials, consult the FDA Code of Federal Regulations for guidance on additional submission requirements before your trial begins.
3. Engage the team early in the process
Clinical trial designs depend on early decisions and strategies. Identify cross-functional team members, including engineering, regulatory, clinical and marketing, to allow experts to contribute in areas of their specialties. All members will have different roles within the clinical trial process.
To ensure you get the needed design feedback for the product, let the cross-functional team know which steps are most important. It’s your job as the expert in design and product functionality to communicate how to effectively use your product.
4. Study planning
As part of the investigational trial plan, study subjects will be identified using inclusion/exclusion enrollment criterial. Terms may differ depending on the content provided, trial photography use and storage, and whether product team members can participate in the trial or enrollment process. Another consideration is whether participants will wear monitors or keep diaries to track activity levels.
Before any data is collected or observations recorded, study-related documents are reviewed and approved by an institutional review board (IRB). The trial investigator or delegate reviews the informed consent document with the participant before enrollment. Subject participant consent must be obtained before the start of any study.
5. Demographics matter
Part of designing a trial is to address the needs of the end-user. For instance, for trials testing a wearable product, consider the participants’ skin and its unique characteristics. Are you allowing people with tattoos to participate? What age groups are involved? What ethnicities are included?
In addition, you need to consider external factors such as skin preparation, whether the weather is humid or dry and how active participants will be.
Work with your clinical team to get a demographic reflective of your customer and from regions where the product will be sold.
6. Keep records and documents secure
Recording documentation, including photography, will make several aspects easier at trial’s end. For instance, if there is an unexpected complication such as an adverse skin reaction, photography may support the adverse event documentation and subsequent timed observations.
7. Stay involved with the team to the end
We often think we’ll understand how people will engage with our product design, but we have no idea what happens when it is in their hands. Being able to see the subjects’ responses to using your product is critical to the final product design.
Observe how the instructions are given to the subject, as well as how the product is applied, especially if the product is to be applied by the user. You may learn things about your product you may not have considered previously such as: Does it catch on a subject’s shirt every time they change? Is the product too small to maneuver for someone who has arthritis?
If you are unable to observe performance in person, have detailed discussions with the clinical study manager on product use.
Clinical trials provide helpful information, so it helps to avoid common mishaps that may occur along the way. If you are interested in learning more about the lessons learned by design engineers when creating wearable medical products, check out this e-book.
Kris Godbey is an application development specialist at 3M with over 35 years in various 3M divisions working in product development and technical support roles.
Robin Huneke Rosenberg is a senior clinical research associate at 3M and currently works in the Health Care Business Group, Medical/Clinical affairs group.
The opinions expressed in this blog post are the author’s only and do not necessarily reflect those of Medical Design and Outsourcing or its employees.