
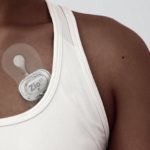
iRhythm’s Zio cardiac monitoring device [Image courtesy of iRhythm]
iRhythm President and CEO Quentin Blackford — the former Dexcom COO who replaced Mike Coyle in September — said his company will need to keep costs under control as it grows to serve tens of millions and then hundreds of millions of patients in the years ahead.
“This means introducing things like automation where it’s possible, reconfiguring processes and streamlining systems and structures across the organization, as well as really rethinking how and where we do the work in this new remote environment that we’re proving can be so successful,” Blackford said today at the J.P. Morgan Healthcare Conference. “Ultimately our goal is to ensure that we have the infrastructure in place to ensure long-term profitable revenue growth.”

iRhythm President and CEO Quentin Blackford [Photo courtesy of iRhythm]
“The big challenge is the fact that we’ve got G&A spend that sits at well north of 50% of every revenue dollar, being consumed by back office and G&A-type activities,” he said. “That’s where we have to get focused to really drive the meaningful leverage into the future, coupled with our cost of sale, the COGS profile of the business. … Both of those areas have increased significantly over the last — call it two years or eight quarters — both on a unit cost perspective and a cost-to-serve perspective.”
Managing those costs will allow iRhythm to invest in innovation and growth, Blackford said. At the same time, some of the savings “gets dropped through to the bottom line and creates the nice financial leverage into the profile of the company into the future.”
“It’s about introducing this operational discipline and just really setting the company up for long-term scalable, profitable growth that affords you those sort of opportunities to invest in growth drivers,” he said.
Blackford also said he hopes for a final reimbursement rule and rate sometime in 2023.
iRhythm stock jumped about 40% after Monday’s positive news, and a BTIG research report projected shares to trade even higher in the quarters ahead.
“Despite the reimbursement uncertainty throughout 2021, [iRhythm] has consistently reported strong sequential volume growth and is working to reduce operating expenses, namely G&A,” BTIG analysts Marie Thibault and Sam Eiber wrote in the report. “The company continues to have several growth drivers, including continued demand for the core product Zio XT, recent traction with Zio AT, future market opportunities in silent AFib, and international expansion.”
With more than 1,600 employees (up from around 1,500 in late 2021), San Francisco-based iRhythm is one of the largest medtech companies in the world, ranking No. 97 on our 2021 Medtech Big 100.




![A photo of the Medtronic GI Genius ColonPro polyp detection system flagging a potential sign of colon cancer during a colonoscopy. [Photo courtesy of Medtronic]](https://www.medicaldesignandoutsourcing.com/wp-content/uploads/2024/04/Medtronic-GI-Genius-doctors-268x170.jpg)