
Michael Gilvarry is the GM of Cerenovus in Galway, Ireland. [Photo courtesy of Johnson & Johnson MedTech]
Gilvarry is the GM of Cerenovus in Galway, Ireland. He founded the Johnson & Johnson Medtech unit’s stroke science research arm and leads the acute ischemic stroke R&D portfolio.
That research led to the development and design of the Embotrap stent retriever device for thrombectomies, which helps stroke patients by removing the clots blocking the flow of blood to their brains.
“How the device design is informed by stroke science is something that we feel is really unique about the device,” he said in an interview. “All of that work we’ve done has led to a very differentiated device design. We’ve demonstrated through testing on the bench and we see in our clinical studies that it’s really led to a device that’s very unique and is yielding very good results and good outcomes for patients.”
Gilvarry spoke with Medical Design & Outsourcing to explain how the Embotrap stent retriever’s design, materials and components work together to treat ischemic stroke. The conversation has been lightly edited for space and clarity.
What’s the typical patient profile for a thrombectomy?
Gilvarry: The patient profile is quite wide-ranging. People often think of stroke as being associated with older people and generally that is the case. About 75% of stroke victims are over the age of 65. But it’s not exclusively older patients. Our devices have been used to treat patients in their twenties and thirties, and unfortunately, it’s common enough depending on the type of comorbidities they might have. The symptoms that somebody develops when they get a stroke are the ones that we’re all familiar with through the public awareness campaigns: the face droop, the loss of power in the arms and [trouble speaking]. That’s caused by a blood clot that normally travels from somewhere else in the body — from the heart, in a large percentage of patients, for example — and that causes a blockage in one of the large vessels in the brain and that triggers the ischemia that leads to the symptoms. The goal of the thrombectomy procedure is to remove the blood clot and restore blood flow to the brain.

The Embotrap stent retriever [Photo courtesy of Johnson & Johnson MedTech]
How is the thrombectomy procedure performed with a stent retriever?
Gilvarry: The device is normally accessed to the vascular system through the groin. In some cases, through the radial artery as well, but it’s predominantly through the femoral artery. The devices are tracked up using a series of catheters. There’s access catheters to navigate around the aortic arch up into the carotid artery. And that’s essentially where the basecamp is set up. Then the thrombectomy devices are tracked further into the vessels in the brain. The Embotrap device is deployed across the clot. And to do that, it’s tracked through a microcatheter less than a half millimeter in diameter that crosses the blood clot. The Embotrap device is placed through the microcatheter underneath the clot. And then the microcatheter is unsheathed and the device opens up inside the blood clot. There’s a wire that goes all the way back outside the body the physician pulls to [withdraw the device,] trapping the blood clot and bringing it back through the access catheter, normally with co-aspiration.
How many passes does it take to remove the blood clot from the vessel?
Gilvarry: It’s removed ideally in one pass. One pass is something we really focused on in the Embotrap device, to get a very efficient clot removal. It’s been shown that good clinical outcomes are positively correlated with a single-pass procedure.
How long does a thrombectomy procedure take?
Gilvarry: It’s very fast. We’ve had procedures that can take less than 10 minutes. It’s a metric that’s widely recorded because they say “time is brain” in stroke. The patients are often awake actually during the procedure, and it’s just remarkable to hear the stories of patients recovering on the table. … One of the things a doctor will often do as soon as the procedure is finished is ask the patient to squeeze their hand and they can tell straight away if they’ve got power back in their arm that the signals are very good, that the patient’s going to recover.
Is thrombectomy a risky procedure?
Gilvarry: Overall, the benefits of this procedure far outweigh the risks. The clinical trials have shown good outcomes increase by 74% compared to tPA clot-busters alone, and the rate of death and disability has been reduced by more than half. There’s a very, very strong risk/benefit ratio here.
How does Embotrap do the job differently than its predecessors?
Gilvarry: We really focused on building features into the Embotrap device that address the various properties of blood clots. The open inlet windows, for example, in the Embotrap device are designed to encourage the clot inside the device. People generically call these devices stent retrievers, but the Embotrap device does not behave like a stent. A coronary stent, for example, is designed to open up a vessel and push everything outward. But the Embotrap outer cage is different. It’s designed with these openings to allow the clot inside the device. Then there is an inner channel, which stabilizes the clot during its removal. It’s almost like a stent within the stent. The inner channel is a closed-cell structure cut from a tiny nitonol tube. … And then the device has a closed end to prevent the clot from traveling all the way through the device as it’s being removed.

A blood clot captured by the Embotrap stent retriever [Photo courtesy of Johnson & Johnson MedTech]
What did you learn about blood clots that helped you design this device to capture them?
Gilvarry: What was remarkable even 10 years ago is how little there was published around the properties of blood clots. And it’s really been a journey of discovery around those properties. Thrombectomy wasn’t the standard procedure so there were very few clots to study even 10 years ago. We started doing tests to define the physical properties as you would with any material. One of them is friction. Trying to understand the frictional properties of blood clots, what we found is that changes substantially both with the composition of the blood clot, but also with how it’s manipulated. If the blood clot is compressed, it can increase friction. That’s one example of many things that we studied and learned and built into the features of the Embotrap device. The device itself has got quite large, open inlet windows, and the intent there is to try and encourage the clot to go inside the device so it’s not overly compressing it, to trap it inside the device and then remove it from the vessel.
How do the materials used in the Embotrap device help with clot removal?
Gilvarry: The device itself is [mostly] made from nitinol, or nickel-titanium alloy. The superelastic and shape memory properties of nitinol make it particularly suited to this device. These vessels are very tortuous. Even compared to coronary arteries, there’s a lot more tortuosity in the pathway to get to the vessels in the brain. So the scale of these is quite small. They’re collapsed down into a catheter that’s less than a half millimeter, and it’s the nitinol that allows it to be collapsed down into that very-low-profile stage in order to be tracked at very, very low forces through the microcatheter up into these vessels. And then once it’s unsheathed, the shape memory properties of nitinol and the superelastic properties help this predefined shape to open up inside the blood clot.
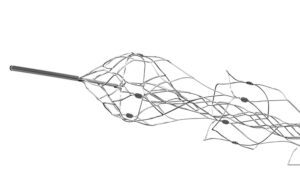
The distal end of the Embotrap stent retriever [Photo courtesy of Johnson & Johnson MedTech]
How do the tungsten radiopaque markers help with the procedure?
Gilvarry: The tungsten markers are embedded in the struts — very, very small scale, but at each section of the device, there are four markers so when the device opens up inside the vessels or indeed inside the clot, the physician can visualize how the device either conforms to the vessel or how it’s interacting with the clot based on these markers. Depending on the length of the device, there are three to five different segments. That’s really important because what that allows is for the device to maintain its connection with the vessel wall as it’s being retrieved [and] helps engage with the clot all the way through the retrieval process.
How is the Embotrap stent retriever manufactured?
Gilvarry: The business end of the device, the nitinol parts, are made by laser cutting nitinol tube into the pattern that we have designed in the Embotrap. We use very-high-frequency lasers to do that because the scale of these are so small. We’re trying to put very fine patterns into very, very small tubes. The device then is heat-treated to set the shape and electropolished to give it the surface finish we need to be used in interventional procedures. It’s a very intricate process to add the radioplaque markers in the device. And then it’s assembled onto the wire, which is made from coated nitinol material. A lot is assembled, then sterilized with EtO and shipped to the customer. … Some of [the manufacturing] is outsourced and some is in-house.
Update: New results detail J&J Embotrap design’s first-pass performance in stroke patients




![A photo of the Medtronic GI Genius ColonPro polyp detection system flagging a potential sign of colon cancer during a colonoscopy. [Photo courtesy of Medtronic]](https://www.medicaldesignandoutsourcing.com/wp-content/uploads/2024/04/Medtronic-GI-Genius-doctors-268x170.jpg)