(Image courtesy of Medovate)
Medtech manufacturer Vygon Group recently announced that it will distribute Medovate’s SAFIRA (SAFer Injection for Regional Anaesthesia) device across 60 countries.
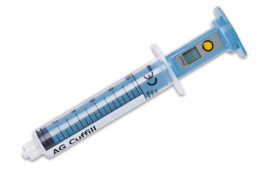
Developed in collaboration with UK National Health Service clinicians, SAFIRA is designed to transform the technique of peripheral nerve blockade into a one-person procedure, giving the anaesthetist full control of the injection at all times. The technology may also improve patient safety by helping reduce the risk of nerve damage, as it is designed to prevent anaesthetic from being injected at high pressures. Furthermore, economic modelling has shown SAFIRA has the potential to help generate significant time and cost savings in a medical setting, according to Cambridge, UK-based Medovate.
A phased launch of SAFIRA in Europe is planned for the end of 2020, with other global markets to follow.
Medovate develops new technologies created through its development pipeline of NHS innovations, with a focus on anaesthesia, airway, critical care and surgery. Vygon is one of the largest independent French medical device companies, supplying more than 205 million products globally per year.
“This agreement with Vygon marks a significant milestone in the launch of SAFIRA across Europe, the U.S. and a number of other markets,” said Medovate managing director Stuart Thomson in a news release. “This will help establish Medovate on the world stage as a recognized medical device developer and manufacturer. In addition, it raises the international profile of the UK NHS and its clinicians, who are key stakeholders in Medovate and inventors of the SAFIRA innovation.”
“This is a very exciting partnership for Vygon,” added John Kerridge, VP of the anaesthesia & emergency business unit for Vygon. “The SAFIRA technology, in terms of its impact on patient safety and clinical end-user efficiency, is in-line with our overall objective of patient/practitioner-based healthcare solutions.”