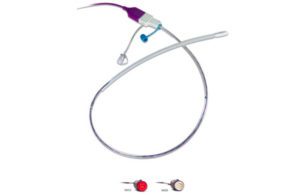
Cardinal Health’s Accu-Trace intrauterine pressure catheter (Image from Cardinal Health)
More medtech companies are experiencing shortages of medical devices due to the sudden closure in February of a Sterigenics sterilization plant.
Officials from Cardinal Health (NYSE:CAH), and Guerbet (EPA:GBT) have written letters to customers indicating that certain devices are already in short supply or may experience shortages, Medical Device & Outsourcing has learned.
The letter from Cardinal Health (Dublin, Ohio) said that the company expects a shortage of its Kendall Accu-Trace intrauterine pressure catheter until early August. This device is placed inside a pregnant woman’s uterus to monitor uterine contractions during labor. The letter from Guerbet (Villepinte, France) said that disposable power injectors used with its Optistar, Optivantage and Illumena contrast delivery systems may experience shortages. Officials from those companies did not immediately respond to requests for comment.
Teleflex Medical OEM (Gurnee, Ill.) expects shortages as well, according to a court filing made in a lawsuit that Sterigenics has instituted against the Illinois Environmental Protection Agency (EPA). The agency ordered the plant to shut down over emissions of ethylene oxide (EO), which is used to sterilize some medical devices and is also highly carcinogenic. Teleflex had seven million devices sterilized per year at the Willowbrook plant, company global procurement director Gregg Twomey said in the filing.
“Specifically, the shutdown will require Teleflex to work with customers to identify alternative products that can be used as substitutes for those sterilized at the Willowbrook Facility,” Twomey said. “Assuming suitable substitute products are identified, our ability to provide such products on a timely basis will depend on current inventory levels of such substitute products, as well as the location of such inventory. In the event suitable substitute products cannot be identified, those products will be unavailable to customers until Teleflex is able to identify and qualify an alternative sterilization facility for those products.”
The Sterigenics Willowbrook plant shutdown affects only one Cardinal Health product, according to company spokeswoman Brandi Martin.
“Cardinal Health representatives can help identify an alternate supplier that can offer a substitute in the interim,” Martin said in an email to Medical Design & Outsourcing. “Cardinal Health is closely monitoring this situation and any impact on our products.”
Twomey did not immediately respond to a request for further comment.
Medtronic (NYSE:MDT) also used the Sterigenics Willowbrook plant to sterilize devices used in minimally invasive surgery, according to Medtronic spokesman Fernando Vivanco.
“Medtronic uses a variety of sources for sterilization across our businesses, both in terms of using multiple sterilization suppliers and multiple geographic locations,” Vivanco said in an email to Medical Design & Outsourcing. “The company believes that this is a transient issue that will affect its Surgical Innovations business in fiscal Q4 2019 and Q1 2020. Medtronic has instituted its business continuity plans and is working to address its sterilization needs through alternate facilities and sources as it works back to full sterilization capacity during the first quarter of fiscal 2020.”
Medtronic won’t know how much the Willowbrook plant shutdown will affect it financially during its fiscal fourth quarter until the quarter ends later this month, he added.
On Friday, the FDA announced that Smiths Medical (Plymouth, Minn.) is experiencing a shortage of its Bivona tracheostomy tube, used by many pediatric patients. The FDA anticipates the tube will be made available again the week of April 22.


