A rapid optimization technique for mitral-valve modeling is applicable to other cardiac structures.
Reza Salari, Thornton Tomasetti Applied Science

Preselected matching points are used to measure the difference between the simulated and target shapes of a diseased mitral valve. (Image courtesy of Thornton Tomasetti)
The medical device industry is making great strides in finite element analysis (FEA), a fundamental tool underlying simulation-guided product development. FEA enables evaluation and prediction of the behavior of implanted medical devices and their interactions with human tissues. Yet going from accurate modeling of normal organ and tissue behavior to patient- and/or disease-specific simulations remains a significant challenge.
When evaluating heart disease with simulation, one approach to creating diseased-state models is to generate 3D models and FEA meshes from patient-specific imaging data such as CT scans and/or MRI. While the associated segmentation software has improved significantly, this process can still be extremely time-consuming. It also may not capture all of the details required for the simulated behavior to match the true diseased state, especially when there is motion involved, as is the case with a beating heart.
An alternative approach that can even be used in conjunction with scan-based segmentation methods is to modify a pre-existing, reasonably representative FEA model to allow the heart’s simulated behavior to match the targeted behavior seen in the diseased state. Our group has developed a shape-matching, inverse finite element framework that can be applied to develop simulations of the mitral valve (MV) that are a closer fit to the behavior seen in diseased states. This has the potential to be applicable to modeling patient-specific organ behavior going forward. The work was presented to the ASME 2018 V&V (Verification & Validation) Symposium.
In the case described here, we started with the Living Heart Human Model (LHHM, from Dassault Systèmes), which provides a baseline FEA model that fairly accurately represents a beating, healthy heart. We created a submodel of the mitral-valve region alone for greater efficiency. To further improve runtime, we developed a proprietary technique that creates a surrogate model from the submodel, harnessing machine learning to optimize results in a matter of minutes instead of the hours that a submodel might take to run. Here’s how we developed this novel methodology.
Setting up the mitral-valve submodel
Located between the left atrium and the left ventricle of the heart, the mitral valve (MV) is made up of two leaflets that contract tightly together with each beat, briefly sealing off the ventricle so it can push oxygenated blood to the rest of the body. When a myocardial infarction (heart attack) occurs, heart-muscle tissue death can negatively affect the function of the chordae tendineae—the many slender ligaments that attach the leaflets to that muscle.
Malfunction of the chordae results in incomplete closure of the leaflets and leakage of blood back into the atrium, or mitral-valve regurgitation. The patient receives less oxygen than normal and becomes out-of-breath and fatigued. Patient-specific computer simulations of this condition can aid clinicians in educating patients, enable surgeons to customize interventions, and help researchers develop new corrective strategies.
To create a diseased-state mitral-valve model, we started with a baseline FEA submodel of a healthy mitral valve taken from the LHHM, which includes all the relevant parameters needed to simulate MV behavior, such as:
- The meshed geometry of the mitral annulus and leaflets (both shape and thickness)
- Mitral-leaflet material properties and fiber orientations
- The number and placement of the chordae
- Chordae material properties
- Length and connection to the leaflet (“zone of influence”), and
- The properties of the associated papillary muscles.
This baseline model is driven by global (full) heart-model boundary conditions, as are the pressures on the bottom surfaces of the mitral leaflets that reflect the pressure difference between the heart’s left atrium and ventricle. But this smaller model saves considerable runtime when compared with conducting the valve analysis inside a full-heart model.
Inverse FEA solution
Some parameters that most acutely affect the behavior of the mitral-valve submodel include leaflet geometry and material properties, chordae length, thickness and material properties, chordae connection to mitral leaflets, etc. Given a diseased-state mitral-valve shape, the question is what adjustments to these submodel parameters are needed in order to most closely represent the behavior of the diseased valve.
To answer this question, where the model output is already known and you are looking to identify what input to your model caused the known output, we turned to an inverse finite-element approach. We adjusted the parameters and updated the submodel in an iterative and systematic way. At each iteration, we compared the results of the updated submodel against the target state to minimize their differences through an optimization process. Iterations continued until the tuned submodel accurately represented the diseased shape.
To keep the model size down, we focused on adjusting the chordae lengths. We used Isight process automation and optimization software to build the inverse finite-element framework. The mismatch between the target and the simulated leaflet shapes was measured through a function that described the difference between the simulated and target positions of a finite number of preselected matching points. The mismatch was minimized through subsequent model iterations by updating the length of the chordae. As the discrepancies were reduced, a closer approximation of the actual disease state was achieved.
Reducing run-time from hours to minutes with a novel algorithm
This mitral-valve submodel analysis, although quite accurate, was also the most time-consuming part of our methodology, taking nearly half an hour per iteration to run, with hundreds of iterations needed to reach the best match of leaflet shape to disease state. We decided to investigate how machine learning might be used to substitute a faster-running surrogate model for our high-fidelity submodel.
Starting from a design of experiments (DOE) setup of our mitral-valve simulation problem, we used machine learning in conjunction with a proprietary algorithm to effectively generate the same submodel output (describing leaflet position) in far less time. The algorithm removed the necessity of re-running the submodel every time there was a change in chordae input, and provided similar results to those from the submodel with minimal loss of accuracy.
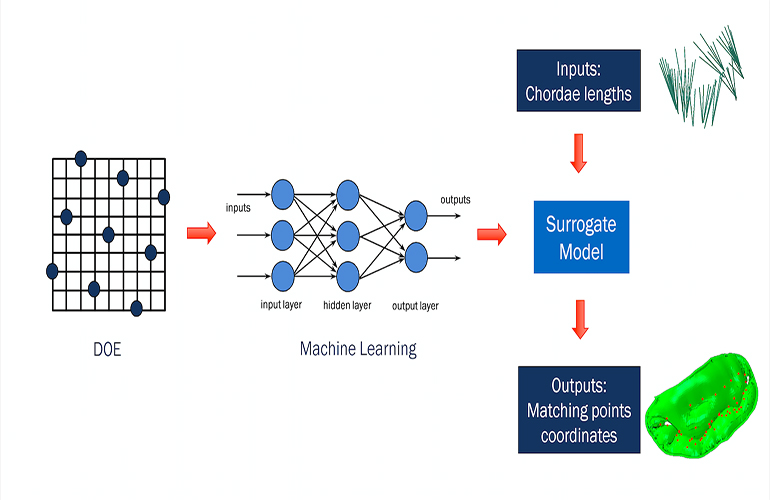 The surrogate model was then dropped into an Isight optimization loop, which ran much more quickly than before. This allowed us to go from initial shape to target shape in just a few minutes, rather than many hours.
The surrogate model was then dropped into an Isight optimization loop, which ran much more quickly than before. This allowed us to go from initial shape to target shape in just a few minutes, rather than many hours.
A widening field of applications
We initially developed this new methodology for mitral-valve modeling, and are now applying our process to the tricuspid valve as well. We are also working to make this kind of time-saving analysis available for other structures, such as the blood vessels around the heart. Starting with CT scans of arteries, we are using our proprietary algorithm to fine-tune blood-vessel models to optimally match artery shapes during heart contraction and relaxation.
Reza Salari is an associate with Thornton Tomasetti Applied Sciences. He has a background in computational mechanics with expertise in finite element analysis, biomechanics, optimization and machine learning.




![A photo of the Medtronic GI Genius ColonPro polyp detection system flagging a potential sign of colon cancer during a colonoscopy. [Photo courtesy of Medtronic]](https://www.medicaldesignandoutsourcing.com/wp-content/uploads/2024/04/Medtronic-GI-Genius-doctors-268x170.jpg)