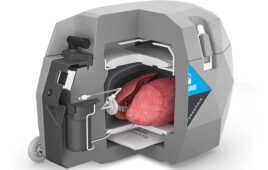
Paragonix Technologies just landed support from the Lung Transplant Foundation to continue developing and commercializing SherpaLung, the latest in its series of specialized organ transport carriers. Braintree, Mass.–based Paragonix launched similar devices for hearts and kidneys within the past several months, according to CEO Bill Edelman.
SherpaLung is not yet approved for sale in the U.S. Paragonix has patents issued and pending for the device.
Based on the current method of lung preservation and transportation, only one out of every five lungs donated in the United States can be used for transplantation, according to the Organ Procurement and Transplantation Network. SherpaLung also provides organ parameter monitoring during storage and transport of donor lungs to recipients for implantation. Lung transplantation is considered gold standard therapy for patients in end-stage pulmonary failure due to idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease.
“The lung transplant field is in critical need of easy-to-use and effective preservation devices for the transport of lungs between organ donation and implantation,” said Lung Transplant Foundation president Jeff Goldstein. “The Paragonix approach to organ preservation is innovative and finally addresses the clinical needs for donor lung transportation. We are so incredibly excited about partnering with Paragonix Technologies to support these efforts.”
The parties did not make public the amount provided by the foundation.
“They decided that they believe the need is so profound and they’re impressed with our technology and our other initiatives in organ transplantation,” Edelman said.
SherpaLung is a single-use, continuous monitoring and validated temperature control product similar in design to Paragonix’s SherpaPak cardiac and kidney transport system.