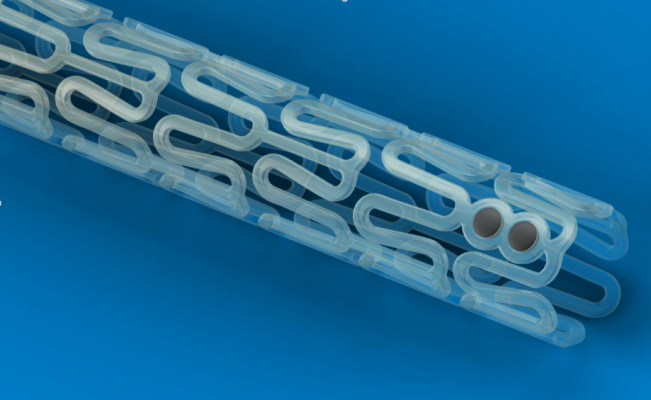
(Credit: Diagnostic and Interventional Cardiology)
Most people have heard about personalized medicine. But how about personalized medical devices?
Northwestern Engineering’s Guillermo Ameer and Cheng Sun have teamed up to use 3-D printing to develop flexible, biodegradable stents that are customized for a specific patient’s body.
“Right now, the vast majority of stents are made from a metal and have off-the-shelf availability in various sizes,” said Ameer, professor of biomedical engineering in Northwestern University’s McCormick School of Engineering and professor of surgery in the Feinberg School of Medicine. “The physician has to guess which stent size is a good fit to keep the blood vessel open. But we’re all different and results are highly dependent on physician experience, so that’s not an optimal solution.”
Supported by the American Heart Association, the research is published online in the journal Advanced Materials Technologies. Robert van Lith, a postdoctoral fellow in Ameer’s laboratory, and Evan Baker, a graduate student in Sun’s laboratory, are co-first authors of the paper.
When ill-fitting stents move in the artery, they can ultimately fail. In these cases, physicians have to somehow re-open the blocked stent or bypass it with a vascular graft. It’s a costly and risky process.
“There are cases where a physician tries to stent a patient, and the fit is not good,” Ameer said. “There might be geometric constraints in the patient’s vessel, such as a significant curvature that can disturb blood flow, causing traditional stents to fail. This is especially a problem for patients who have conditions that prevent the use of blood thinners, which are commonly given to patients who have stents. By printing a stent that has the exact geometric and biologic requirements of the patient’s blood vessel, we expect to minimize the probability of these complications.”
To create these customized stents, Ameer worked with Sun to adapt a 3-D printing technique, called projection micro-stereo-lithography, to fabricate stents using a polymer previously developed in Ameer’s lab. The technique uses a liquid photo-curable resin or polymer to print objects with light. When a pattern of light is shined on the polymer, it converts it into a solid that is then slowly displaced to cure the next layer of liquid polymer. The printing technology allows the team to fabricate a stent that precisely matches desirable design characteristics.
Sun’s 3-D printing technique, known as micro continuous liquid interface production (microCLIP), has several advantages. First, it is extremely high resolution. With the ability to print features as small as 7 microns, it is perfect for printing stents, which have very fine mesh dimensions and can be smaller than 3 millimeters in diameter. Second, it has the ability to print up to 100 stents at a time, producing them faster and potentially cheaper than traditional manufacturing methods. Third, it’s fast, printing a 4-centimeter stent in a matter of minutes.
Although current stents are made with metal wire mesh, Ameer used a citric-acid based polymer previously developed in his lab. The resulting stent is flexible, biodegradable, and has inherent antioxidant properties. Drugs can also be loaded onto the polymer and slowly released at the implantation site to improve the healing process in the blood vessel wall. Ameer has previously shown that the polymer can be engineered to inhibit clot formation when applied to vascular grafts. The stent’s strong but biodegradable allows it to exercise its mechanical function during the vessel’s initial dilation and slowly dissolve as the re-opened blood vessel recovers.
“In theory, it’s safer because the patient doesn’t have permanent foreign metal devices in the body,” Ameer said. “If, for any reason in the future, the surgeon needs to go back in to that location in the vessel, they can. There’s not a metal stent in the way.”
Current biodegradable stents are made from plastics similar to those used for sutures. They are not as strong as wire mesh and can take longer than metal stents to fully expand when deployed. To compensate for this weakness, the plastic stents are strengthened by increasing the thickness of their struts relative to that of a metal stent. Ameer’s 3-D printed stent, however, can be fabricated with the thinner profile of traditional metal wire stents, so it is more compatible with the body.
Ameer and Sun, associate professor of mechanical engineering, imagine a future process whereby the dimensions of a patient’s vessel are obtained using standard imaging techniques available at hospitals, and a stent is then printed on site to exactly fit the vessel’s dimensions, packaged, and given to the surgeon for implantation. Next, Ameer plans to investigate how long it takes for his biodegradable stent to break down and absorb into the body. His team also aims to investigate innovative stent designs to improve their long-term performance.
“Not only can we customize the stent for a patient’s blood vessels,” he said, “but we can create all new types of patient-specific medical devices that could make the outcomes of surgical procedures better than what they are today.”




