(Image from Duke University)
TransMedics (NSDQ:TMDX) announced today that the doctors at two hospitals performed the first U.S. heart transplants using donor hearts that were resuscitated using the company’s organ care system (OCS).
The transplants from donation after circulatory death (DCD) took place at Duke University Hospital in Durham, N.C. and Massachusetts General Hospital in Boston. In a DCD procedure, the donation process can start only after the heart has stopped beating. Currently, DCD donors are not considered for heart transplantation due to the potential for injury once the heart stops beating and the inability to assess heart viability using cold static storage, according to the Andover, Mass.-based company.
A donation after circulatory death differs from the more traditional heart donation after brain death (DBD), in which the heart will continue to beat with mechanical support. In a DCD, the family has withdrawn life support and death has been declared due to the cessation of circulatory function. Only about 30% of available DBD hearts are transplanted due to the limitations of cold storage, the company noted.
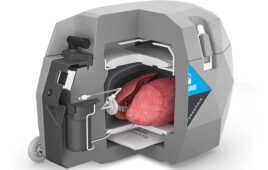
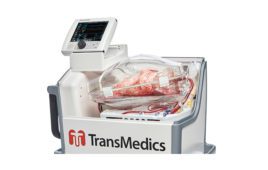
TransMedics’ heart OCS is designed to keep the organ in a warm, oxygenated, normal-beating state, allowing the surgeon to treat it and assess its viability for transplant before transplantation. The OCS heart system is the only medical device that has enabled DCD heart transplantation to become a clinical reality, and to date has also been used in the UK and Australia.
“This procedure has the potential to expand the donor pool by up to 30 percent,” said Dr. Jacob Schroder, who performed the procedure at Duke, in a news release. “Increasing the number of donated hearts would decrease the wait time and the number of deaths that occur while people are waiting. It’s important to conduct this clinical trial to determine whether those outcomes are realized,” Schroder said. “We are grateful for the courage and generosity of both the donors and recipients.”
The DCD heart transplantation milestone at Duke occurred Sunday after a donated heart was deemed viable for transplant. The recipient, a military veteran who received his heart through the Mission Act, is recovering well, according to the university hospital.
The OCS heart system is being evaluated for use in DCD heart transplants across several U.S. heart transplant centers in an pivotal clinical trial.
“This a critical milestone for U.S. heart transplantation that could enable a significant increase in heart transplant procedures to help many end-stage heart failure patients,” said TransMedics president & CEO Dr. Waleed Hassanein in a separate news release. “We are looking forward to the continued progress of this important clinical trial and the continued adoption of OCS as the next standard of care for heart, lung and liver transplants.”
TransMedics went public in May 2019. TMDX was trading up 1.98% at $18.06 in mid-morning trading.