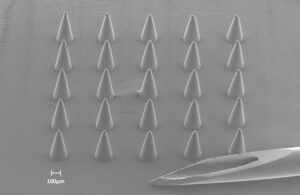
These 3D printed microneedles — viewed through a scanning electron microscope — are pictured next to a traditional 29-gauge hypodermic needle. A human hair is approximately as wide as the 100 micron scale marker in the image. [Image courtesy of Mayo Clinic]
Microscale 3D printing has the potential to revolutionize medical device development.
Seth Hara and Renc Saracaydin, Mayo Clinic
The Mayo Clinic Division of Engineering is an embedded engineering team that provides engineering support and service for researchers and clinicians throughout the enterprise. To meet their needs, the engineering team has embraced the use of microscale 3D printing.
Microscale 3D printing in medical device development is still relatively new. As this technology continues to mature, the field will continue to find new and exciting opportunities to advance the practice of medicine.
As the name implies, microscale 3D printing is the use of additive manufacturing techniques to produce structures that have features as small as a few microns. To put everything into scale, a human hair is around 100 µm (microns) in diameter; that is one-tenth of a millimeter or four-thousandths of an inch. The ability to 3D print at such a small scale opens the door to a variety of applications in the biomedical sector.
This technology can additively manufacture 3D microfluidic chips to be used for diagnostic blood tests, cell sorting, drug discovery and organoid synthesis, to name a few applications. It can be used to fabricate drug delivery systems such as microneedle arrays to allow the self-administration of drugs or vaccines painlessly and effectively. Additionally, it enables manufacturing of porous architectures or scaffolds that can be used to guide the growth and proliferation of cells for regenerative medicine applications such as the treatment of spinal cord injuries. Beyond these cutting-edge applications, microscale 3D printing enables the fabrication of connectors, fixtures, and other auxiliary components that are essential for medical devices.

Seth Hara is a Mayo Clinic Division of Engineering senior engineer and manager of the Microfabrication Laboratory. [Photo courtesy of Mayo Clinic]
Microscale 3D printing takes shape
Over the last decade, a surge of 3D printing technologies have become commercially available. Some of the most common are material extrusion, vat photopolymerization, powder bed fusion, and material jetting. Even though these technologies are capable of manufacturing relatively large components with great repeatability and reliability, they often struggle to scale down the features below a couple hundreds of microns.
However, with advancements in optics, microelectromechanical systems (MEMS), and materials science, new technologies capable of microscale 3D printing have emerged. Proprietary names for some of these technologies are micro-stereolithography (µSLA), micro-digital light processing (µDLP), projection micro stereolithography (PuSL), and two-photon polymerization (2PP).
The working principle of these technologies is similar (except for 2PP, to be explained later). A viscous photopolymerizable resin is exposed to visible or ultraviolet (UV) light based on the geometry of the desired part. As light interacts with the resin, it cures the resin and turns it into a rigid polymer. Specifically, the energy provided by the UV light creates reactive species called free radicals that covalently bond to monomers and/or oligomers to initiate the photopolymerization reaction.
As more molecules are bonded together, the reaction propagates until it is terminated. This reaction leads to long polymer chains that can be interconnected depending on the type of resin used. This process is repeated for each layer until the final part is realized. What allows these technologies to print in the microscale is their ability to highly localize the photopolymerization reaction through the use of customized resin chemistry and high optical resolution ranging from 1 to 5 µm.
On the other hand, 2PP operates in a different regime and utilizes resin chemistry that requires two femtosecond pulses to photopolymerize an extremely localized focal volume. This allows structures to be printed in free volume with submicron resolution, trading off the larger build volumes that can be accomplished with the former technologies.

Renc Saracaydin is working on microscale additive manufacturing as a graduate microfabrication intern at Mayo Clinic. [Photo courtesy of Mayo Clinic]
Microscale 3D printing’s challenges
As with any emerging technology, there are many challenges and obstacles that must be addressed to realize microscale 3D printing’s full potential. New materials must be developed to leverage advancements in optical technology and processes must be tuned so that photopolymerization is contained to small volumes while adequately forming secure bonds.
Once parts are printed, post-processing presents another round of challenges. As with any resin-based printing, uncured resin must be removed from cavities and lumens. This is often accomplished by immersing the part in a solvent bath and agitating the solution. Microscale prints, however, can be vulnerable due to their small features and so agitation must be done carefully — if at all — to prevent damage and breakage. This is made even more difficult by the intricate patterns and small volumes designed into microscale parts, which often require more agitation to properly remove all uncured resin. These competing requirements highlight the critical need for process tuning and design strategies for microscale prints.
Microscale 3D printing for medical device development adds an additional level of complexity to processing considerations. The materials that enable printing on the microscale often do not have a long history of biocompatibility and must be thoroughly tested for the intended application. Furthermore, the printing process itself must be evaluated and controlled to verify that the material properties of the final part are repeatable and safe. If the printed part is to be used in an application that requires sterilization, strategies must be put into place in the design stage to ensure that the selected sterilization process will not negatively impact the material properties of the part and that all surfaces are properly sterilized.
This is an exciting chapter for both additive manufacturing and medical device development. Microscale 3D printing is opening doors to new possibilities and providing tools for researchers and engineers to develop the next generation of medical devices.
As the challenges facing this new technology are addressed, microscale 3D printing will likely be a common and enabling fabrication tool in this highly innovative field.
Renc Saracaydin is pursuing a master’s degree in materials science and engineering at University of California, Los Angeles, and is working on microscale additive manufacturing as a graduate microfabrication intern at Mayo Clinic.
Seth Hara is a Mayo Clinic Division of Engineering senior engineer and manager of the Microfabrication Laboratory. He has a background in bioMEMS and received his Ph.D. in biomedical engineering from the University of Southern California.
The opinions expressed in this post are the author’s only and do not necessarily reflect those of MedicalDesignandOutsourcing.com or its employees.




![A photo of the Medtronic GI Genius ColonPro polyp detection system flagging a potential sign of colon cancer during a colonoscopy. [Photo courtesy of Medtronic]](https://www.medicaldesignandoutsourcing.com/wp-content/uploads/2024/04/Medtronic-GI-Genius-doctors-268x170.jpg)