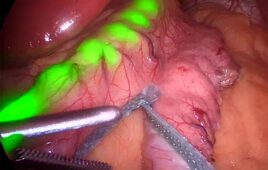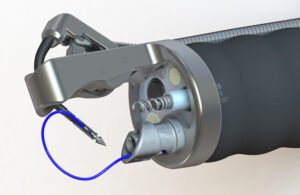
The Apollo Endosurgery OverStitch endoscopic suturing system [Image courtesy of Apollo Endosurgery]
The procedure is called endoscopic sleeve gastroplasty (ESG), and the device that made it possible is the OverStitch endoscopic suturing system.
Austin, Texas–based Apollo Endosurgery first won 510(k) clearance for OverStitch in 2008, with successive clearances for improved designs over the years. Most recently, in July the FDA granted de novo clearance for Apollo Endosurgery’s Apollo ESG, Apollo ESG Sx, Apollo REVISE and Apollo REVISE Sx systems. They’re the first FDA-authorized devices for ESG and endoscopic bariatric revision procedures.
Medical Design & Outsourcing spoke with Apollo Endosurgery Chief Medical Officer Dr. Christopher “CJ” Gostout and R&D VP Tom Neudeck earlier this year — before the Boston Scientific deal was announced — to learn more about the new devices and procedures.
“We [have] the only intralumenal suture system that does full-thickness running and interrupted stitching,” Neudeck said. “There’s nothing else out there.”
“There is no competition” that provides consistent, full-thickness suture placement, Gostout added.
This conversation has been lightly edited for space and clarity.
How and why was Apollo Endosurgery founded?

Apollo Endosurgery CMO Dr. Christopher “CJ” Gostout [Photo courtesy of Apollo Endosurgery]
Your initial focus differed from today’s devices, is that right?
Gostout: In 2005, NOTES (natural orifice translumenal endoscopic surgery) hit the scene. … One of the Apollo group projects other than a suturing device was to establish transgastric cholecystectomy procedures. The Apollo group had completed several animal labs that demonstrated it was certainly feasible to do transgastric operations of some kind, whether it’s an appendectomy, a cholecystectomy, or even a fallopian tube ligation. A group from India did a transgastric appendectomy and that launched NOTES. There was a meeting with representatives from the two endoscopy societies, the ASGE (American Society for Gastrointestinal Endoscopy) and SAGES (Society of American Gastrointestinal and Endoscopic Surgeons). We convened in New York City, formed a working group called NOSCAR (Natural Orifice Surgery Consortium for Assessment and Research) to push forward NOTES. So Apollo was formed thinking that we were going to create medical devices that would enable NOTES procedures and at the top of the pyramid of our devices would be our suturing device. NOTES fell by the wayside despite physician enthusiasm. Insurance companies received it as highly experimental. The medical device companies were not interested in creating any ancillary devices to facilitate NOTES procedures. Consequently, with a dependable suturing device in hand, the gastric reduction project was resurrected at Mayo.
Where did you go from there?
Gostout: Through a series of animal models — starting out with a pig stomach, then a dog stomach and then a baboon stomach — I identified a suture pattern that would effectively reduce the gastric volume by about 75–80% and looked like a sleeve, mimicking a sleeve gastrectomy. Encouraged by the results, we initiated a clinical pilot study at Mayo.
You had your doubts?
Gostout: To be honest with you, my thoughts were, “I don’t know if this is going to last. Maybe a couple of weeks, maybe not.” Our first patient was a success, losing weight and becoming a distance runner. The patient subsequently developed symptomatic gallstones and had to have a gallbladder removal. During the gall bladder removal, the surgeon commented that the stomach was impressively reduced with the full-thickness sutures present, seen from the outside. I thought, “Wow, we did it. We hit the home run.” … We completed the other cases for the study, and we were getting the same results. And I thought, “Well, this procedure’s here to stay.” And then it caught on. The technique became modified to add efficiency to the procedure and ensure long-term suture retention.
What sort of patients is this ESG procedure meant for?
Gostout: It’s a very viable, scalable procedure for weight loss that fits very nicely in the spectrum of opportunities to help guide obese patients throughout their life of weight loss. It fills the gap between standard weight loss efforts with diet and exercise, pharmacotherapy and traditional surgery. Our ESG system can be used within the BMI range of 30-50.
How does the ESG procedure work?
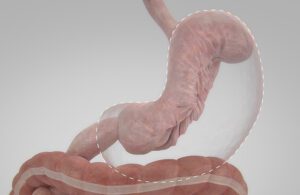
Endoscopic sleeve gastroplasty (ESG) shrinks the stomach with intralumenal suturing. [Image courtesy of Apollo Endosurgery]
How much weight do patients lose after ESG?
Gostout: It doesn’t compare to the sleeve gastrectomy by any means. Patients will not lose the same amount of weight. They should be expected to experience total body weight loss ranging from 15% to 18% or more. There will be significant improvement in associated comorbidities such as type 2 diabetes, hypertension, and metabolic syndrome. A patient can get the benefits of a surgical procedure — not as much dramatic weight loss, but significant weight loss — and have improvement in some comorbidities with a safer, lower-risk procedure.
How did Apollo Endosurgery develop the OverStitch device?

Apollo Endosurgery VP of R&D Tom Neudeck [Photo courtesy of Apollo Endosurgery]
How did you make the turn toward commercialization?
Neudeck: We received additional funding at that point and split our resources. One part of the business supported our clinical work with our Gen 1 device. And the majority of the R&D people focused on developing our Gen 2 platform, taking into consideration the learnings and user feedback from the field. The second generation of Overstitch is the device which is commercially available today. We launched it just over 10 years ago. And the first ESG procedure was done with the Gen 2 device by Dr. Gostout in 2012.
And that’s when you outsourced your supply chain?

Apollo Endosurgery’s OverStitch Sx for single-channel scopes (left) and OverStitch for dual-channel scopes (right) [Image courtesy of Apollo Endosurgery]
How important is the OverStich device’s curved needle arm?
Gostout: Traditional suturing employs a curved needle. Every attempted suturing device functions as a sewing machine using straight needles. The Apollo Group wanted a curved needle to add the versatility of being able to place a stitch wherever you want as opposed to having to align tissue into a sewing machine. The OverStitch device needle tip has a T-tag design to the needle which fits into a curved driver arm. That curved arm simulates the ergonomics of hand suturing. That’s the beauty of our device, the curved needle arm.

Apollo Endosurgery’s OverStitch device pulls stomach tissue toward it to avoid puncturing other organs. [Image courtesy of Apollo Endosurgery]
What did Apollo Endosurgery learn about the stomach that you’d like to share with our readers?
Neudeck: The stomach is probably one of the toughest environments for developing implantable med devices. The constant movement of the stomach, and the gastric fluid’s acidity make it a very harsh environment. And pH and diet is different from patient to patient. Blood in comparison is a very well-characterized fluid and very similar across patients, which makes it easier to develop benchtop models and test methods to do a lot of the early engineering working in the lab. Trying to simulate the gastric environment is really difficult. The stomach is a big muscle. If you do something to it, it responds over time to space-filling or -restricting implants. And the physiology of the gastric tract, with it being a strong peristaltic pump, we have seen a lot of other companies trying to develop obesity devices over the years but often failed because of the unique environment, because everything starts to migrate in the stomach.
Gostout: One thing that’s an absolute is durability provided by the ability to place full-thickness sutures. I had conducted research centered around creating new working spaces inside the GI tract wall beneath the lining, which has become referred to as third-space endoscopy. A variety of applications were studied and included trying to secure magnets in the wall of the esophagus and stomach, placement of drug-eluting materials to treat localized diseases and even access the surface of the heart. These research efforts demonstrated if any foreign material is not placed full thickness, the body extrudes it over several days. It’s impressive how your body deals with foreign materials, and it’s this full-thickness aspect that’s absolutely a must for durability of a suture-based weight loss procedure.
This post was updated with additional information and commentary from Neudeck and Gostout in January and February 2023.

![A photo of the Medtronic GI Genius ColonPro polyp detection system flagging a potential sign of colon cancer during a colonoscopy. [Photo courtesy of Medtronic]](https://www.medicaldesignandoutsourcing.com/wp-content/uploads/2024/04/Medtronic-GI-Genius-doctors-268x170.jpg)