
AVS is developing the Pulse system for peripheral intravascular lithotripsy. [Image courtesy of AVS]
The name of the company’s peripheral intravascular lithotripsy (IVL) system is Pulse. The Boston-based startup could start enrolling patients in an investigational device exemption (IDE) study as soon as next year.
AVS Chief Operating Officer Sean Gilligan joined the device developer after nearly 30 years with Boston Scientific, including 15 years as VP of program management and R&D. In an interview, he credited advances in balloon catheter technologies for improving performance at the smaller sizes needed to access peripheral and coronary vessels.
“The evolution in terms of treating intravascular disease — both peripheral and coronary — it’s night and day from 20, 30 years ago, when you look at these big, bulky balloon catheters that just did basic stuff,” Gilligan said in an interview with Medical Design & Outsourcing. “Heart disease is still very prevalent, but many people are fortunate to get to the hospital in time and can be treated and within a few days are back to normal life.”
Treating intravascular calcium has remained a challenge, even with the evolution of balloons, stents, drug-coated stents and atherectomy devices.
“No two lesions are the same, no two patients are the same,” Gilligan said. “Calcium is not perfectly uniform. And because it’s hard to treat, it takes a lot of patience, a lot of cost in terms of devices, etc. There are technologies that do some of the work but potentially can’t do as good a job. And I think that’s largely why Shockwave has taken off so quickly.”
Like IVL leader Shockwave Medical, AVS designed its technology to clear blocked blood vessels by cracking the calcium without causing too much damage to the vessel wall. Shockwave’s technology generates short bursts of low-energy, high-voltage pulses that cause cavitation inside their balloon catheter and breaks up the calcium.
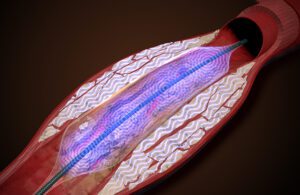
AVS designed the Pulse system to expand the artery while it breaks up the calcium to restore blood flow. [Illustration courtesy of AVS]
“Our concept is not based on an electrical stimulus,” Gilligan said. “What we’re doing is we’re creating a high-frequency pressure pulsation on the fluid pathway. … We’ve filed intellectual property based on our technology. And I know there are lots of different companies looking at different ways of trying to do the same thing. Many of them are chasing the electrical stimulation paths.”
That non-electrical approach could offer advantages when it comes to procedure flexibility, ease of use, overall system size and portability, Gilligan said.
“Hospitals don’t want to buy 10 systems. They want to be able to move equipment between cath labs,” Gilligan said. “Cath labs are busy. There’s a lot of information flowing when a physician is doing a procedure. We want to make sure the ergonomics and the handling are as good as they can be to simplify the workload on the cath lab staff, but also making sure that we can, as an example, minimize the number of devices that might be used in a procedure for cost and time.”
What’s next for AVS

AVS Chief Operating Officer Sean Gilligan [Photo courtesy of AVS]
“Things are looking good to achieve those milestones right now,” Gilligan said. “And then beyond that, it’s really down to how fast the clinical trial enrolls, gathering that data, and submitting the 510(k) to the FDA for approval.”
AVS is also working on its coronary IVL platform in parallel, Gilligan said.
“Some parts of the system overall would be leveraged from the peripheral platform, so that gives us a little bit of head start. … The coronary platform is smaller in terms of profile than the peripheral ones, so we need to do some work to make sure that’s optimized prior to starting first-in-human study.”




