Biosense Webster U.S. President Nikki Sidi [Image courtesy of Biosense Webster]
Electrophysiology is “an incredibly vibrant space,” said Nikki Sidi, but access remains a challenge.
Nikki Sidi, the U.S. president of Johnson & Johnson’s Biosense Webster, can look at her own company as one of those paving the way in electrophysiology (EP). Its recent milestones include multiple atrial fibrillation (AFib) mapping catheters, including ablation and mapping devices.
Sidi has worked across a number of businesses at J&J, including leading U.S. marketing for Biosense Webster. She took on the post of U.S. president at Biosense Webster in December 2022 after a stint with J&J Vision.
Her return to Biosense Webster — and her return to AFib treatments — has been nothing short of “amazing,“ she said in an interview with Medical Design & Outsourcing. Biosense Webster competes in a market that also includes Boston Scientific, Medtronic and Abbott — with excitement growing around the potential of new pulsed-field ablation treatments and more.
“There’s so much that is happening, so much innovation that’s coming,” Sidi said. “With Biosense being the market leader, it’s great to be at the helm of that and seeing what else we can do, not just from an innovation perspective, but also from removing some of the barriers that impact our physicians and customers alike to get access to care.”
During September — AFib Awareness Month — Sidi and Biosense Webster want to continue to battle AFib and the barriers preventing people from receiving proper care for the disease.
“It’s incredible because we’ve been at this for decades, but the penetration of patients accessing this treatment is still relatively low,” she said. “There’s still a lot of work to do, and a lot of innovation to come to hopefully unlock that as well.”
Health disparities build up AFib barriers

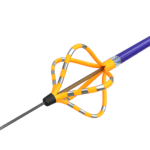
The Optrell mapping catheter, one of Biosense Webster’s tools for treating AFib. [Image courtesy of Biosense Webster]
Sidi referenced a Journal of the American College of Cardiology article stating that Black and Hispanic patients have a higher prevalence of AFib risk factors. These include high blood pressure, diabetes and obesity. Black patients are 30% more likely to have high blood pressure and 60% more likely to have diabetes. And Hispanic patients are 1.2 times more likely to be obese compared to other ethnic groups,, Sidi said.
“Just the fact that they’re disproportionately more prone to having these types of risks, that means a more disproportionate amount affected by AFib,” Sidi explained. “There’s a huge opportunity for us to impact them.”
Most alarming to the Biosense Webster executive: Only about one-third of Black AFib patients are likely to be aware that they have AFib.
“It’s important to get the awareness out if we want to eliminate some of these inequities,” Sidi said.
According to Sidi, a lack of trust leads to roadblocks for people in these communities who need care. She said it centers around the simple fact that people want to see physicians and receive care from people who look like them, have the same experiences they do, have gone through some of the same challenges and understand their circumstances.
“For us, that just means we need more diversity in medicine in general so these physicians reflect the patients they serve,” Sidi said. “That’s ultimately our goal.”
Biosense Webster’s diversity initiatives

The QDot Micro catheter used in treating AFib [Image courtesy of Biosense Webster]
The company serves as the founding sponsor of Heart Rhythm Society’s Growth and Leadership Opportunities for Women in Electrophysiology (GLOWE). This program helps women navigate the challenges in EP, as Sidi said the field has one of the lowest percentages for female representation.
This year, Biosense Webster also collaborated with HRS on a program for Black electrophysiologists called Growth and Leadership Opportunity for Black Electrophysiologists (GLOBE).
“Black EPs have traditionally been underrepresented at the executive level, in healthcare in general and certainly within EP,” Sidi said. “Putting in those programs to help create an environment where these minority groups feel supported has been really critical and core to the way we’ve approached it.”
Biosense Webster also invested in STEM programs to tap into the student population and help guide them toward EP. Last year, the company awarded 10 students with a scholarship through a program with the Association of Black Cardiologists (ABC). Sidi said the program helps students participate in leadership groups and gives them an opportunity to “get a flavor for what electrophysiology is.”
The company also aims to provide tools and resources for people with AFib. The best way to address the condition, Sidi said, is to ensure that people are educated on what their options are.
“Ultimately, as a newly diagnosed patient, we want them to understand that AFib is a progressive disease,” she explained. “If it’s not treated early, it can get worse. And the treatment options are more effective when done earlier.”
Biosense Webster has a website dedicated to helping patients learn about their options that includes patient testimonials. The company also announced new materials online that have been translated into 10 different languages to widen accessibility.
“We’re making sure we can get the most people, being inclusive in the way we approach them and hopefully, ultimately impact the care that they get,” Sidi said.
Related: J&J’s Biosense Webster used RWE for expanded indications — and you can, too