The Children’s National Health System recently announced the finalists of their Third Annual Pediatric Surgical Innovation Symposium hosted by the Sheikh Zayed Institute for Pediatric Surgical Innovation. The competition is intended to bring attention to the needs of pediatric patients in having medical technology developed specifically for them and to bring about actual innovations for that effort. The two winning teams of the eight finalists will be announced this Friday and will each be awarded $50,000.
While we await announcement of the winners, MDT reached out to the teams to offer them the opportunity to speak about their innovation and the process involved in its development. The first team to respond was Healthcare Unbound. This team created a noninvasive therapy solution for chest deformities in children. Rutwik Shah, MD, MTM, cofounder of Healthcare Unbound, participated in the interview.
Sean Fenske: Please briefly explain your technology and how it can affect healthcare.
Dr. Rutwik Shah: Our wearable chest vest works on a simple but effective technology — using a balloon brace that allows patients with pectus carinatum (or pigeon chest) to non-invasively treat their condition.
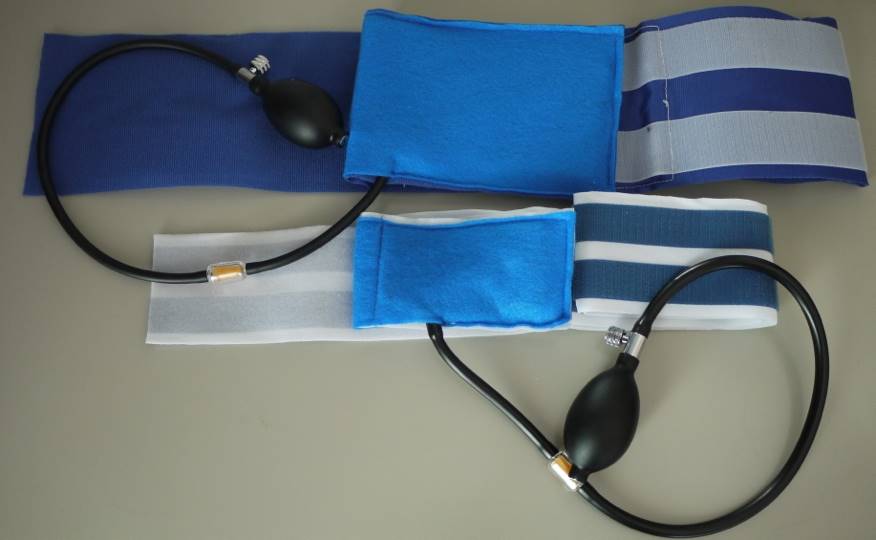
Healthcare Unbound has developed a wearable chest vest to address the medical condition pectus carinatum (pigeon chest).
Fenske: How does it work?
Dr. Shah: The balloon in the brace is connected via a valve to an air pump. After wearing the brace, the user can pump air into the balloon, which exerts positive pressure on the deformed chest and pushes it inwards, allowing correction of the deformity.
Fenske: What was the inspiration for developing it?
Dr. Shah: The inspiration for developing this device came from our patients. Many of them find surgery an unviable solution and the current braces are extremely uncomfortable to wear, resulting in markedly reduced compliance. We also wanted to develop a brace that would be affordable and low on maintenance for patients in the developing world to use.
Fenske: What challenges have you encountered with regard to development?
Dr. Shah: Truly speaking, our biggest challenge was to not over engineer and create something fancy. We set out with an aim to create a technology that can be seamlessly used by anyone, especially the young children who would be using our vest. At the end of the day, we did not want our patients to feel as if they were wearing a medical device, but simply an additional layer of clothing. On the technical side, we had to improve upon our valve design to prevent air from leaking from the balloon so the patients could also wear the vest at night while sleeping.
Fenske: Have you explored the regulatory aspect of medical device development yet?
Dr. Shah: Although we haven’t applied for regulatory approval yet, we are well aware of the path we need to tread. Fortunately for us, our device would fall under the Class I medical device category, which has predicates in the market. This makes it easy for us to obtain FDA approval in the next few months. We additionally have help from UCSF and other regulatory consultants.
Fenske: What’s the most significant thing you’ve learned about medical device development during this experience?
Dr. Shah: We learned a few important lessons along the way.
First, getting the concept down to a real prototype was a tricky task as we were trying to design something while consciously keeping the user experience in mind, which for us was seven- and eight-year-old kids. While the kids would not give us detailed feedback, they certainly would tell us if they hated using something and they weren’t diplomatic!
While designing a medical device or technology for kids, the dictum “less is more” holds very true. We are now keeping this in mind for the other projects we are working on.
Fenske: Why did you enter the Annual Pediatric Surgical Innovation competition?
Dr. Shah: While money and visibility are the obvious answers, one of the main reasons we entered this competition was to meet like-minded people who take it upon themselves to develop solutions to problems they face rather than complain or wait for others to take the lead. We think this competition is a great way to create a community of innovators who deeply care about improving pediatric healthcare.
Fenske: What have been your thoughts of the competition experience so far?
Dr. Shah: While it may be early to comment on this, we believe the organizers have done an outstanding job of bringing the best teams and judges for this event. We sincerely look forward to interacting with all of the attendees.
Fenske: After the competition, what’s next for your company and/or technology?
Dr. Shah: After this, we plan to push our project forward through the approval process. We would also continue to work on other projects and ideas, some of which will emerge during our interactions with others at the competition.
Fenske: Any other comments or thoughts you’d like to share?
Dr. Shah: Developing medical devices is a hard task and it takes many people to take an idea to success. We hope to network with other teams and explore the opportunity of working with some of them. After all, it takes a village to raise a baby.
On the other hand, being based in the Bay area, we have an unprecedented advantage of working in the heart of the innovation hub of the world. We would be equally willing to help other teams develop and test their projects and help them raise funds through our contacts.




