Combining diagnostic imaging technologies with the design freedom of additive manufacturing has opened up new opportunities in prosthetics, enabling custom patient devices and improving the effectiveness of diagnosis, planning, surgery and clinical outcomes.

This osteoinductive material may eliminate negative effects related to current bone fillers, such as cements and bio-cements. (Image courtesy of GE Additive)
Surgeons mainly use custom implants when bone geometry is not within the dimensional range of standard implants, when there are special requirements due to disease, or simply when a tailor-made solution enables a better clinical result.
For the successful use of the custom prostheses, inter-professional cooperation and communication between the orthopedic surgeon and the implant designer are key. The implant designer may not be familiar with anatomopathological, epidemiological, surgical or resection/reconstruction procedures, while the orthopedic surgeon may not have an in-depth understanding of the process of producing a physical additively manufactured model.
Additive manufacture of customized prostheses
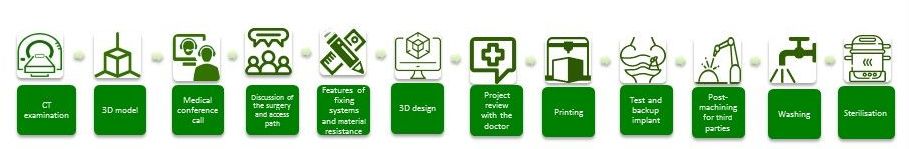
To develop customized prostheses, it is necessary to go through a series of steps.
From the outset, this process requires close cooperation between the surgeon responsible for the procedure and an implant manufacturer’s engineering team. The first step is a CT scan to make it possible to build a 3D model of the specific anatomical characteristics of the patient. A conference call is then held with the doctor, where the intervention is discussed, and access routes identified. Designers then determine the characteristics of the fixing systems, considering the resistance of the material.
The prosthesis is designed, and then reviewed with the surgeon, for all aspects of feasibility. Finally, the prosthesis is printed, often with a backup copy, to support any unexpected events or problems. The prosthesis usually does not require any special post-treatment other than washing and final sterilization, which can take place at the source or in an autoclave at the destination hospital.
Driving advances in cranioplasty
Leveraging its experience distributing a range of medical-surgical devices to hospitals, public and private healthcare facilities and now with additive technologies, Sicily-based MT Ortho is developing innovative solutions for orthopedic surgery, oncological orthopedics, neurosurgery and maxillofacial surgery.
This has led to the development of a new line of custom cranioplasty prostheses, now in use throughout Europe. The use of additive technology in cranioplasty makes the prosthetics process easier and more precise. In addition, the characteristics of the technology make it possible to achieve an optimal structure for osseointegration. By virtue of the speed and precision of the technology, it is also easier to carry out the so-called demolition/reconstruction operations of cranioplasty in a single step, according to the company.
These interventions are based on a different surgical strategy that allows the most precise planning of the intervention, for which MT Ortho provides not only the prosthesis but also the cutting templates, following the precise mapping of the intervention area by a CT scan.
In surgery, the removal of the area affected by disease and the insertion of the cranial prosthesis can take place simultaneously, drastically reducing the post-operative hospitalization and recovery time and the risk of infection. This is particularly important for interventions in sensitive areas such as the ocular orbit or the cranio-maxillofacial region.
Custom solutions for bone cancer patients
Until recently, only standard, conventionally manufactured prostheses, or in limited cases custom prostheses, were available for patients with bone tumors.
Bone cancer is one application area where additive manufacturing can offer advantages, especially when combined with digital image processing and artificial intelligence technologies, making it possible to prepare a 3D intervention plan through the fusion of several CT images.
The full design freedom offered by additive technology allows for the manufacture of custom prostheses that consider deformation and the need to adequately distribute loads. Using additive technologies, it is possible to perfectly reconstruct the bone anatomy of patients after demolition surgery performed for the removal of a tumor.
Looking back and ahead
MT Ortho had two decades of experience in the Italian clinical-hospital market for standard prostheses before it started to embrace additive manufacturing in 2014. That year also marked a series of arrivals at the company; a recent graduate engineer, Simone Di Bella, who had specialized in additive manufacturing, and two GE Additive Arcam electron beam melting (EBM) machines.
“Our goal was to become not only a distributor but also a manufacturer of medical devices,” DiBella said. “And our vision was to achieve this by creating new, innovative devices with unique features that were only possible by using additive manufacturing and were more compatible with the human bone than metals on the market at the time.”
The team at MT Ortho initially focused on the production of custom prostheses, for neurosurgical applications (custom cranioplasty) and oncological orthopedics (mega-prostheses reconstruction). At the same time, the company launched several projects to obtain the European CE mark for several devices in the field of neurosurgery.
Now MT Ortho is working to develop a kyphoplasty implant for the treatment of vertebral collapse. This device could make it possible to replace current bone fillers, such as cements and bio-cements, with an osteoinductive material eliminating all possible negative effects related to the current technology in use, according to the company.