Now more than ever, it’s critical for drug-delivery systems to address usability needs and patient safety. Here are three key takeaways to ensure that patients stay central to the development process for drug-delivery devices.
Kennedy Clark, West Pharmaceutical Services
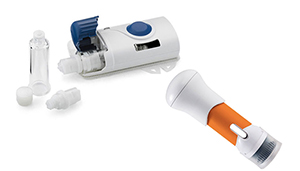
West Pharmaceutical Services is betting that an embrace of human factors engineering and experiential design will allow its drug delivery devices such as its SmartDose platform (left) and the SelfDose patient-controlled injector (right) to stand apart from the crowd. [Images courtesy of West]
For the treatment of many chronic conditions, such as Crohn’s disease, diabetes, multiple sclerosis and rheumatoid arthritis, drug administration continues to move from a doctor’s office to self-administration in the patient’s home. As a result, the market demand for patient-centric delivery platforms has increased dramatically. If patients are not comfortable with – or have difficulty using – the technology, adherence levels can drop.
It’s more critical than ever for drug-delivery systems to address patient safety and usability needs. Therefore, drug-delivery platform providers are more vigorously exploring innovation and technology to build patient centricity into the design of self-administration systems and stay ahead of market demand. This requires conducting extensive research into three patient-facing areas: Human factors, device usability and patient needs.
Human factors
To design a drug-delivery system that will truly resonate with patients, the first step is understanding their behaviors and motivations. This requires conducting research that will drive innovation in the design and development processes, creating a solution that works in a variety of situations. Methods for pinpointing behaviors and motivations include:
Collecting qualitative data: Interviews, ethnographic observations, contextual inquiries and concept evaluation.
Gathering quantitative data: Questionnaires, in-person surveys and user-based performance testing.
Analyzing and synthesizing outputs: Affinity diagramming, product adoption road maps and habits and ideal scenarios.
Identifying ergonomics: Human error and risk analysis, usability testing and heuristic analysis (encouraging a person to use the device on his/her own).
Device usability
Once human factors are identified, it’s crucial to gain a deeper understanding of how appropriate a drug-delivery platform is for patients. Typically, the most impactful data is gathered through interviews and observations in the proper context. Seeing the user in the midst of daily distractions – such as caring for an aging parent and interacting with children, pets, ambient noise, temperature and lighting – all help human-factors experts better understand how the patient will use a device. Testing can be broken down into four major components:
Physical abilities: Anthropometry (the measure of bodies, such as height or the size of hands), biomechanics (what can be accomplished physically) and sensory abilities (vision, hearing and tactile sense).
Cognitive abilities: How people process information, the capabilities of memory, the manner in which humans learn new things and how habits are developed.
State of being: The general health of the expected user, disease states and co-morbidities, mental and emotional states and motivation for learning new things.
Experiences: Educational background, knowledge of a particular disease state and lifelong experiences with objects that will guide behavioral interactions with any delivery system.
Patient needs
A third critical aspect to developing a patient-centric self-administrative platform is defining what they need out of the technology, beyond successfully delivering the therapy. Research can uncover information that will personalize the system and help increase adherence. Those needs include:
Expected needs: Meaningful to patients; direct observation inside the user’s environment is an effective way to document them.
Expressed needs: Simple for users to articulate; “think-alouds” and other narrative techniques are best to determine expressed needs.
Exciting needs: Typically delights patients; they often do not think about the features as technically possible.
Taking human factors, device usability and patient needs into account is critical to designing delivery systems that patients can and want to use. In doing so, drug-delivery platform providers will not only meet the unique needs of pharmaceutical partners, but more importantly, also ensure the device’s user interface is safe and effective for the intended population and environments.
Kennedy Clark is a Senior Director leading the Global Market Development team for West proprietary devices. He has earned both MBA and Bachelor of Science in Industrial Technology degrees.