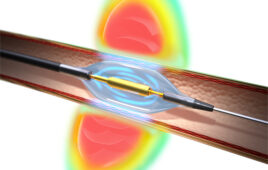
Medtronic’s Symplicity Spyral renal denervation catheter delivers energy to the nerves leading to the kidneys, which help regulate blood pressure. [Image courtesy of Medtronic]
The panel unanimously said the minimally invasive catheter system is safe, but was nearly split on efficacy. With six members voting that the benefits outweighed the risk, six voting the other way and one panelist abstaining, panel chair Dr. Richard Lange cast the deciding vote to recommend against FDA approval.
It’s not necessarily the end for Medtronic’s effort to secure approval of its Symplicity Spyral multi-electrode RDN catheter and Symplicity G3 radiofrequency generator to reduce blood pressure in certain hypertension patients. From here, the FDA will consider the panel`s recommendation and let Medtronic know whether it agrees and whether additional information is needed.
“We’re looking forward to working together with the FDA and taking into account all of the comments that we heard today,” Medtronic SVP and Chief Scientific, Medical, and Regulatory Officer Dr. Laura Mauri said after the vote.
The stakes are high for Medtronic, which has been developing the minimally invasive technology for years in the belief that it could be a billion-dollar business and provide relief to millions of patients worldwide, reducing heart attacks, strokes and other serious events tied to high blood pressure.
The same FDA review panel yesterday supported approval of competing RDN technology developed by Otsuka Medical Devices’ ReCor Medical. If the FDA agrees with the panel’s recommendation on the safety and efficacy of ReCor’s system, it could be the first RDN therapy to be approved for use in the U.S.
ReCor’s clinical trial met its primary endpoint for efficacy, while Medtronic’s RDN trials have failed to hit that target — though analysts have said they don’t think that will prevent Medtronic from winning FDA approval.
More than 1 billion people suffer from hypertension globally, but only around 40% are receiving active treatment. Of those, around one-fifth report feeling like their symptoms are under control.
Medtronic has said its Symplicity Spyral RDN reduces blood pressure by around 5-10 mm Hg, and that a reduction of just 2-5 mm Hg can reduce cardiovascular events by 10%.
Related: Pay drops for Medtronic CEO and the median employee; bonus plan changes
FDA review panel questions Medtronic on RDN

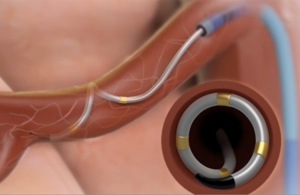
Medtronic’s Symplicity Spyral device is a multi-electrode radiofrequency catheter. [Image courtesy of Medtronic]
Ben Saville, director and senior statistical scientist at Berry Consultants, said he believed Medtronic “introduced some bias in this analysis” but that it does not likely affect the high-level conclusions of the study.
Reviewers expressed concerns that study outcomes may have been influenced by analytical bias and that blinded patients may have determined whether they received the RND therapy. One reviewer called attention to a “lack of race and other demographic information.”
“That certainly would be a big problem for publishing in most of our peer-reviewed journals in this day and age, especially for a question where there’s so much relevance of race and ethnicity to the clinical problem,” said Dr. Matthew Corriere, professor of cardiovascular surgery at the University of Michigan.
“The effect of renal denervation in the African-American population is an important one to consider,” added Dr. Bram Zuckerman, director of the Office of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health.
Dr. Eric Bates, a cardiology professor at the University of Michigan, asked why Medtronic has not yet published its data despite presenting results at the American Heart Association’s meeting in November.
Vanessa DeBruin, senior director of clinical research at Medtronic, said the paper is still in review.
“The hard question is why was it not a simultaneous publication and why nine months later have we not seen the data published?” asked Bates, later continuing, “My concern is had the report been published, the spin on the technology in the last six months in preparation for this meeting might have been muted compared to what I’m reading in the lay and medical press.”
Medtronic responds
“It is true that we have not been able to collect and represent information on race on all of our subjects. … Half of our study enrollment in on- and off-med was conducted outside the U.S, and in that situation we are not permitted by regulations to disclose race and ethnicity,” Mauri said.
But in the studies, she said, 39% of U.S. patients were Black, which is more than the share of Black people who are U.S. residents.
Mauri said Medtronic considered the importance of “representing under-represented populations in our study, as well as understanding the impact in our clinical program related to renal denervation, and we’re very interested, obviously, in the impact of renal innovation in important populations in the United States, including the Black population.”
The off-med group showed “consistent findings within U.S. Black Americans versus non-black Americans with a similar magnitude of reduction, a relatively similar effect in the sham arm, and no significant p-value for interaction,” Mauri said. “This is important because the off-med study is[ the] simplest way for us to really understand that there’s a true biologic effect of renal denervation.”
She said Medtronic will continue to collect additional information on RND for Black Americans.
Dr. David Kandzari, Piedmont Heart Institute’s chief scientific officer and director of interventional cardiology, treated the first patient in Medtronic’s Spyral trial back in December 2021 and today answered questions on Medtronic’s behalf. He explained why the study showed a higher-than-expected reduction in blood pressure for sham patients who did not receive the RDN therapy.
“Consistently across the program — independent of the observational global Symplicity registry, but supported definitely by it — we see consistent themes of reductions in office systolic and diastolic blood pressure [and] ambulatory systolic and diastolic blood pressure with renal denervation therapy in the context of quite extremely favorable safety,” Kandzari said. “In the expansion phase, we saw quite a remarkable imbalance, an asymmetry in medication changes with the sham patients significantly increasing their medications compared to the renal denervation group and the renal denervation patients significantly decreasing their medicines in comparison to the sham control group.”
FDA review panel discussion and votes
The FDA review panel took issue with the Medtronic Symplicity Spyral RDN system’s proposed indications for use (IFU), saying the study lacked evidence to support it.
The proposed indications are “for the reduction of blood pressure in patients with uncontrolled hypertension despite the use of anti-hypertensive medications or in patients in whom blood pressure lowering therapy is poorly tolerated.”
“There’s a huge disconnect between this IFU and what was studied,” said Dr. Keith Allen, director of surgical research St. Luke’s Hospital of Kansas City, Missouri.
Allen would later vote yes on the system’s safety, no on efficacy and no for benefit/risk.
“While this is a very safe procedure, the efficacy was mild at best,” he said, later continuing, “Sometimes we like to think that the threshold for efficacy should be lowered when the device is very safe. We still have to think of the overall healthcare burden, particularly of devices that are going to be used in patients and whether it’s appropriate.”
Dr. Randal Starling, professor of medicine at Cleveland Clinic’s Heart, Vascular and Thoracic Institute, voted yes on all three questions. “Hypertension is not effectively treated with current tools, and more tools are needed to treat blood pressure,” he said.
Also voting yes on all three questions was Dr. Robert Yeh, director of the Center for Outcomes Research in Cardiology at Boston’s Beth Israel Deaconess Medical Center, who said the off-med trial “clearly demonstrates at least some effectiveness.”
“There will be patients who would benefit,” he said. “I do think it will behoove the community to identify who those patients are. But I thought making the device available will help the community figure out who those patients are and accelerate treatment of patients in need.”
Bates also voted yes on all three questions, but said his vote was not to recommend the technology for the general public, but rather a vote to continue research into which patients are most likely to benefit.
“We don’t want to lose the technology if we can make it work better and pick out the sub groups where it might work,” he said.
Lange, who as chair did not vote on safety or efficacy because those votes did not need a tie-breaker, said he would have voted yes and no, respectively. However, he said he might have supported the Medtronic RDN system on efficacy under a different indication, but “for the indications proposed, I don’t think it was proved to be effective.”
As to his tie-breaking vote against the system’s risk/benefit considerations, he said he agreed with the other review panel members who cast votes against recommending FDA approval of Medtronic’s RDN system.
“We’re faced with a negative trial, unfortunately. And we can try to explain it away. But in the end, it’s a negative trial,” Lange said. “And so I can’t honestly look patients in the eye and tell them that I believe that the benefit outweighs the risk in the patient population at large.”
Related: After Medtronic’s latest setback, what’s next for Symplicity Spyral RDN?
Today’s FDA review panel is available for replay below: