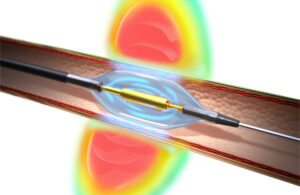
The Recor Medical Paradise ultrasound RDN system uses a water-filled balloon catheter for 360-degree sonification to ablate overactive nerves in the walls of the renal arteries. [Illustration courtesy of Recor Medical]
Recor Medical held a companywide town hall for employees to celebrate winning premarket approval (PMA) for their Paradise Ultrasound RDN system. While they were celebrating, the team learned that the very first commercial procedure had just been completed.
“After a pretty long journey of many years — the rigorous clinical trials that we ran, and all the work that they’ve done — they were actually seeing, finally, this wonderful result of a PMA,” Recor President and CEO Lara Barghout said. “We hit the ground running immediately. … It was such an incredible, emotional moment for the team to be talking together at a townhall and then getting the news about the patient.”
In an interview with Medical Design & Outsourcing, Barghout discussed the long road to approval, the technology that made it possible, and how Recor accomplished what medtech giants could not over the past decade.
“It’s incredible to have the first renal denervation therapy launched through a company with this passion and technology. … We also want to get it to as many patients as we can,” she said. “And it was great to see that we were able to do that so quickly after approval.”
Dueling RDN technologies
Recor won a race that Medtronic — now the largest medical device manufacturer in the world — has been running since buying RDN developer Ardien in 2011. Boston Scientific was a contender with its Vessix RDN technology, but bowed out after a failed trial in 2018.
The stakes are huge. More than 1.2 billion people have hypertension worldwide, but most don’t have it under control, causing heart attacks, strokes, kidney damage and premature deaths. Medtronic estimates renal denervation could be a multibillion-dollar business.
“Getting a renal denervation therapy to market is one of the biggest wins that the world of devices has seen in a really long time,” Barghout said.
Medtronic and Recor designed their competing systems to ablate overactive nerves between the brain and the kidneys that help regulate blood pressure. RDN therapy uses minimally invasive catheters inside the renal arteries to apply energy and calm those nerves in the artery walls, lowering a patient’s blood pressure.
Recor seemed lined up for approval in August when medical and statistical experts on the FDA’s Circulatory System Devices Panel of the Medical Devices Advisory Committee determined the Paradise Ultrasound RDN system was safe and effective. One day later, the same experts voted that the risks of competing technology from Medtronic outweighed its benefits.
Recor’s first-of-its-kind ultrasound RDN system hit all of its primary endpoints in its clinical trials. While Medtronic’s clinical trials hit their safety targets both in 2014 and 2022, they missed their primary endpoints on efficacy.
Medtronic’s Symplicity Spyral RDN system uses a self-expanding radiofrequency catheter with four electrodes to apply that energy.
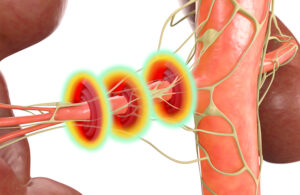
The Recor Medical Paradise ultrasound RDN system delivers 360-degree sonications to ablate overactive nerves inside the renal arteries, lowering a patient’s blood pressure. [Illustration courtesy of Recor Medical]
“The most important thing as the denervation procedure is being done is to be able to reach the nerves in the renal artery to make sure there’s been denervation and that the therapy is actually effective,” Barghout said. “Having a 360-degree sonication is one of the biggest differentiators besides the fact that it’s an ultrasound wave.”
Medtronic congratulated Recor for its FDA approval in a statement shared with MassDevice, calling it “a significant milestone in the field of RDN and for patients and physicians.”
“Medtronic welcomes innovation in the field of renal denervation as it provides further validation of the therapy as a complementary treatment option in reducing blood pressure for patients, in addition to medications and lifestyle changes,” the company said. “Medtronic remains confident in the therapy and continues to have productive discussions with FDA regarding our PMA submission. We look forward to hearing soon from FDA.”
The FDA approved Medtronic’s RDN system less than two weeks later.
Recor’s believers

Recor Medical CEO and President Lara Barghout [Photo courtesy of Recor Medical]
One of the things she’s learned about Recor’s employees in her first year at the helm is how passionate they are about the technology they’ve been developing for so long.
“They are big believers that it actually is effective. One of the things that continue to drive them is the confidence they have in the fact that this technology is actually going to yield phenomenal results for patients,” she said. “It’s simple because the theory behind the therapy — and if you look at how our product is designed — it’s extremely natural to know that this is going to be effective.”
That kept them pushing toward the finish line and lifted them over their biggest hurdle: designing the clinical trials to demonstrate the system’s value.
“Creativity, being able to be consistent, be rigorous, and be patient about recruiting the right patients and continuing to support the protocol that they all aligned on with our physician partners is what really continued to drive them,” Barghout said. “There were three randomized, sham-controlled studies that we did and the minute that the first study resulted in positive outcomes — our SOLO trial — that’s when they knew they just had to finish it off.”
Each small milestone and continued confidence in the therapy gave them “the breath to continue on,” she said.
In 2018, venture capital firm Sofinnova Partners sold Recor to Otsuka Medical Devices, which provided financial stability to keep device development and clinical trials on track even through COVID-19 pandemic disruptions.
“The continuous breath that they had from the Otsuka side to continue to support the team was also a pretty significant portion of how the team continued to keep going,” Barghout said.
Trial design
Because Recor’s Paradise system hit its endpoints in all three trials, “the potential of not having an FDA approval was extremely low in our books,” Barghout said.
“Our goal was to continue to drive until we get to an FDA approval,” she said. “There was no backup plan, and if we were not able to meet that goal in those three clinical trials, we probably would have run more — but it was not necessary.”
She credited the Recor team with developing “solid” trial protocols and for their specific patient selection criteria. The involvement of medication was a challenge for Recor’s trials and hypertension device trials more broadly.
“Mainly what really is needed is for patients to adhere to the medication protocol, whether it’s an onmed or an offmed trial, and to adhere to the follow-up that’s needed after the procedure is done,” Barghout said.
To improve patient compliance, clinical trial subjects received a single pill combining three common antihypertensive medications. Intake observation and urine sampling ensured patients consistently took their meds, ensuring clean data to compare Paradise uRDN against the sham.
And it can be a challenge to find patients willing to participate in a sham-controlled trial because they might not want to be part of the control arm that doesn’t get the treatment.
“The protocol was rigorous, and the patient selection was rigorous, and the team continued to drive according to the plan,” Barghout said.
What’s next
Recor is planning for growth to get more doctors on board and more patients treated. Some of the first procedures were conducted by cardiologists who participated in the clinical trials. Others had been waiting for FDA approval to use the system for the first time.
Barghout wouldn’t disclose exactly how many employees Recor has, but said it’s between 300 and 500. They’ll be joined by new colleagues not only on the commercial side, but also new hires in innovation and R&D, for the clinical team, and infrastructure.
“The first thing is we continue to drive for commercialization, but also we have a huge passion for innovation,” Barghout said. “We’re going to continue to innovate in the world of hypertension therapy, we’re going to continue to innovate in creating and developing data to prove that this therapy is an important part of the world of healthcare and that it can help patients live a healthier life.”
She also sees potential for more innovation in the sympathetic nervous system and treating other chronic diseases with denervation.
“I’m always inspired when I talk to physicians after they treat their patients to understand how the patient’s journey was and how would they be after the procedure,” Barghout said. “That, for me, is what Recor is all about. We are a mission-driven organization. We think about patients first, and we think about everything else after that. I’m really excited to see the team with all the hard work that they’ve done, that we are able to hit all the goals that are in front of us.”
More: Recor Medical CEO Lara Barghout on leadership lessons and what she looks for when hiring
This post was first published on Nov. 15, 2023, and updated on Dec. 21, 2023, with more information about clinical trial design.







![A photo of the Medtronic GI Genius ColonPro polyp detection system flagging a potential sign of colon cancer during a colonoscopy. [Photo courtesy of Medtronic]](https://www.medicaldesignandoutsourcing.com/wp-content/uploads/2024/04/Medtronic-GI-Genius-doctors-268x170.jpg)