Recalled Product:
- HeartWare Ventricular Assist Device (HVAD)
- Serial Numbers: All sterile un-implanted stock in inventory with serial numbers prior to HW25838
- Product Code: 1103
- Manufacturing Dates: July 31, 2014 to March 30, 2016
- Devices Recalled in the U.S.:105 units
(Credit: HeartWare)
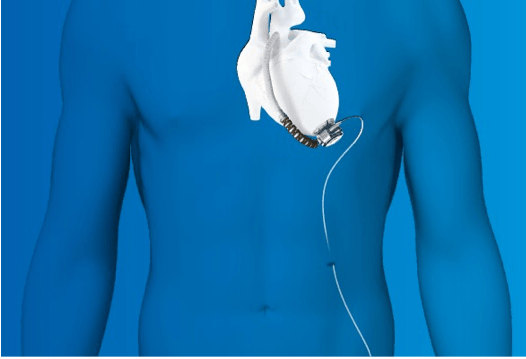
Device Use
The HVAD helps deliver blood from the heart to the rest of the body. It is used in patients who are at risk of death from end-stage left ventricular heart failure and who are waiting for a heart transplant. The system includes a pump implanted in the space around the heart (pericardium) and a controller that controls the speed and function of the pump.
The HVAD is designed for use both in and out of hospital settings, including during patient transport.
Reason for Recall
HeartWare Inc. is recalling the HVAD pumps due to a design problem with the driveline connector. The driveline is a tube that connects the HVAD’s pump to the external controller and power source. Contamination of the driveline may result in fluid or other material entering the pump and causing electrical issues or pump stops that may lead to serious adverse health consequences, including death.
Who May be Affected
- Patients receiving cardiac support using the HVAD system
- Health care providers and caregivers monitoring patients with a HVAD system
What to Do
On August 17, 2016, HeartWare Inc. sent an “Urgent Medical Device Recall Letter” to affected customers. The letter instructed consumers to:
- Identify affected HVADs in hospital inventory
- Complete and return the “Acknowledgement Form” attached to the letter
- Return affected products to HeartWare Inc.
- After returning the affected products, complete and return the “Completion Form” to a HeartWare representative no later than 2 months from the date on the letter
Contact Information
Health care providers who have questions should contact their HeartWare representative or contact HeartWare Inc. at cs@heartware.com or 1-877-367-4823 with any questions related to this recall.
Date Recall Initiated:
August 17, 2016
Additional Resources:
Firm Press Release
How do I report a problem?
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program either online, by regular mail or by FAX to 1-800-FDA-0178.




