[Image from Alan Calvert on Unsplash]
The researchers, who conducted work at UL’s Bernal Institute, published their findings in Biomaterials Research.
According to a news release, they developed new hybrid biomaterials. They turned to nanoparticles built on existing practices in tissue engineering. As a result, they successfully synthesized the materials to promote repair and regeneration following spinal cord injury.
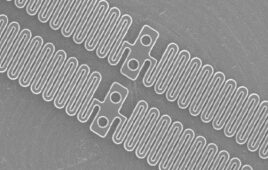
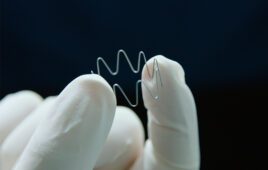
Maurice Collins, associate professor of the School of Engineering at UL led the team. So, too, did lead author Aleksandra Serafin, a PhD candidate at UL. Their research used a new kind of scaffolding material and a unique new electrically conducting polymer composite. This promoted new tissue growth and generation.
“Spinal Cord Injury remains one of the most debilitating traumatic injuries a person can sustain during their lifetime, affecting every aspect of the person’s life,” said Collins. “As there is currently no widely available treatment, continuous research into this field is crucial to find a treatment to improve the patient’s quality of life, with the research field turning towards tissue engineering for novel treatment strategies.”
The team cited growing interest in electroconductive tissue-engineered scaffolds. This emerged as a result of improved cell growth and proliferation from exposure to a conductive scaffold.
Serafin said limitations exist with the PEDOT:PSS commercially available polymer in biomedical applications. That polymer relies on the PSS component to allow for water solubility. When implanted in the body, though, it displays poor biocompatibility.
“This means that upon exposure to this polymer, the body has potential toxic or immunological responses, which are not ideal in an already damaged tissue which we are trying to regenerate,” said Serafin. “This severely limits which hydrogel components can be successfully incorporated to create conductive scaffolds.”
Overcoming limitations
The researchers developed novel PEDOT nanoparticles (NPs) to overcome that limitation. Synthesis of these NPs allows for the tailored modification of the surface of the NPs. The approach helps achieve the desired cell response. This increases the variability of which hydrogel components may be incorporated.
“The introduction of the PEDOT NPs into the biomaterial increased the conductivity of samples,” the researchers said. “In addition, the mechanical properties of implanted materials should mimic the tissue of interest in tissue-engineered strategies, with the developed PEDOT NP scaffolds matching the mechanical values of the native spinal cord.”
Researchers evaluated the biological response of these NPs with stem cells in-vitro and in animal models of spinal cord injury in-vivo. They observed “excellent” stem cell attachment and growth on the scaffolds. These tests also demonstrated greater axonal cell migration toward the site of spinal cord injury and lower levels of scarring and inflammation compared to the injury model which had no scaffold.
“These results offer encouraging prospects for patients and further research into this area is planned, said Serafin. “Studies have shown that the excitability threshold of motor neurons on the distal end of a spinal cord injury tends to be higher. A future project will further improve the scaffold design and create conductivity gradients in the scaffold, with the conductivity increasing towards the distal end of the lesion to further stimulate neurons to regenerate.”
The Irish Research Council, in partnership with Johnson & Johnson, funded the project. The Irish Fulbright Association also contributed, enabling a research exchange to the University of California, San Diego. Faculty at the UL Science and Engineering and Health Research Institute provided support, too.