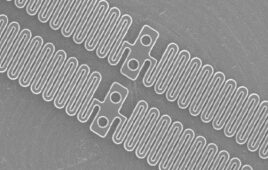
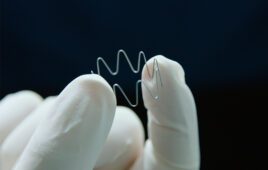
Honeywell’s Spectra MG Bio medical fiber [Photo courtesy of Honeywell]
High-strength, lightweight medical fiber helps make surgical procedures more effective.
Zachary Murnane, Honeywell
The first minimally invasive surgery (MIS) — an endoscopy — dates back hundreds of years. In orthopedics, it has a history of a century or more.
Uptake has accelerated more recently, and has grown fast across a wide range of surgeries in the last few decades. One published analysis of MIS described the expansion as “exponential since the introduction of laparoscopic surgery in the late 1980s.”
MIS is the new standard. In orthopedics, MIS is now widely used for arthroscopies and even full joint replacement for not just knees, but hips, wrists, shoulders and elbows. From gastroenterology to cardiothoracic and heart surgery, pediatric to urogynecology, MIS has surged.
According to one estimate, the market value of MIS surpassed $60 billion as of 2020. By 2030 it is estimated to increase more than a third to $94.4 billion, a compound annual growth rate of 4.7%.
MIS is in a period of accelerating growth. That’s not surprising: the benefits are well recognized by medical professionals and the public alike. Smaller incisions mean less pain, fewer complications, less scarring, and shorter hospital stays and recoveries. They also mean a lower total cost of care.
Given a choice, few would want a larger wound requiring more recovery time, but realizing the benefits of MIS depends on a successful surgery. In part, we need high-quality medical devices produced with materials designed to maximize the benefits of MIS procedures.
Form and function
The rise of MIS poses challenges for medical device companies. Smaller incisions require more precise instruments and smaller medical devices to facilitate surgery. At the same time, requirements for high strength with low stretch, superior flex and bending fatigue performance remain necessary. This applies to Class II devices like catheters and Class III devices such as part of a heart valve. In orthopedics, shrinking incisions do not diminish the need for strong materials.
As with traditional surgery, the qualities and properties of the medical device can have a significant impact. One striking example is the rising use of colored sutures, another trend we’ve seen recently. Colored fibers in sutures can create patterned combinations to aid visibility and suture management in more complex surgeries. That can be particularly valuable when working within the confines of the smaller incisions of MIS, helping promote faster, more effective and potentially safer surgery.
Materials matter
Medical devices are critically important, and the history of suture materials is even longer than that of MIS. It spans from silk and catgut used over three and half millennia ago right up to the present day, to the synthetic materials of the last half-century.
Some forms of ultra-high molecular weight polyethylene (UHMWPE) fiber can be 15 times stronger than steel and three times stronger than polyester while still being ultra-lightweight. This enables strong sutures with a small diameter/footprint. It also provides superior resistance to chemicals, fatigue and abrasion compared to conventional polyethylene. Look for medical-grade UHMWPE with ISO 13485 certification, the highest quality management standard for the medical device industry.
UHMWPE fiber is available in hues of color for high visibility. That visibility — along with UHMWPE fiber’s high strength, light weight, biocompatibility and non-absorbability — makes this material ideal for use in orthopedic sutures or other medical applications where stark visual contrast can aid in making the procedure more effective.
Such developments don’t just support the increasing preference for and prevalence of MIS. Lighter, stronger, high-performance materials will also be critical to supporting the emerging trends in corrective surgeries, from robotics that reduce procedure durations to smart implants that flag dangerous bacterial presence. UHMWPE will play a crucial role in all of these.

Zachary Murnane is a senior research and development applications manager for Honeywell’s Spectra business, which produces Spectra MG Bio fiber. [Photo courtesy of Honeywell]
The opinions expressed in this post are the author’s only and do not necessarily reflect those of MedicalDesignandOutsourcing.com or its employees.