
Medtronic Chief Technology and Innovation Officer Ken Washington (left) and Medtronic Endoscopy Chief AI Officer Ha Hong pose with a scope used with the GI Genius Intelligent Endoscopy Module at the company’s facilities in Santa Clara, California. [Photo by Hardy Wilson for Medical Design & Outsourcing]
In recent interviews, Medtronic executives discussed how artificial intelligence is leading to new or improved devices. They also offered insights that can help device designers and engineers across the medtech industry take advantage of the rapidly advancing technology.
“We’re harnessing the power of AI today for use in clinical decision support, creating new indications, and delivering personalized treatments,” Medtronic Chair and CEO Geoff Martha said at the 2024 J.P. Morgan Healthcare Conference. “… I believe we’re uniquely positioned to advance AI and medtech.”
Medtech is one of the most exciting fields for applying AI because “it directly affects the patient’s life,” Medtronic Endoscopy Chief Artificial Intelligence Officer Ha Hong said in an interview with Medical Design & Outsourcing.
“The good news is you don’t have to invent the science of AI. That’s a solved problem,” Medtronic Chief Technology and Innovation Officer Ken Washington said in the same interview with Hong. “So what we have to do is organize the data and make it easier for our engineers and our scientists who design and do the R&D in our business units and our operating units to create the next generation of products that are going to be powered by these AI technologies.”
Related: AI basics from Medtronic Chief Technology and Innovation Officer Ken Washington
Medtronic AI products
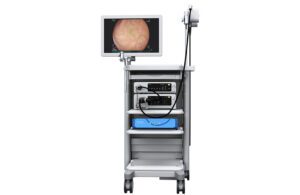
Medtronic’s GI Genius intelligent endoscopy module [Image courtesy of Medtronic]
“In the real world, we’re finding 50% are missed that the AI is picking up without any false positives,” Martha said. “It’s really phenomenal.”
Martha envisions GI Genius becoming the standard in colonoscopy, and said Medtronic is expanding the platform to enable additional AI applications for gastrointestinal procedures.
GI Genius is Medtronic’s best-known artificial intelligence product, but Martha highlighted four others. For example, Medtronic’s AI-enabled AiBLE (pronounced “able”) digital ecosystem for neurosurgery helps surgeons plan, perform and analyze spinal and cranial procedures.
Medtronic Diabetes uses machine learning for automatic insulin delivery in the MiniMed 780G System, which features a Meal Detection Technology algorithm that Martha called “the secret sauce.”
Medtronic’s Touch Surgery Enterprise uses AI to process and annotate videos of procedures for immediate review by the surgeon on their phone, helping to improve training and compliance.

Medtronic CEO and Chair Geoff Martha [Photo courtesy of Medtronic]
“The unlock here was AI,” Martha said. “We were picking up all the AFib and then some. [Physicians] didn’t want any false positives. They’re looking for 100% sensitivity and specificity, and the unlock was AI [to] eliminate those false positives.”
More from the 2024 JPM Healthcare Conference: Medtronic cuts suppliers and manufacturing sites, CEO says in supply chain and operations update
Medtronic’s AI insights and advice
Those Medtronic AI products “are just the tip of the iceberg,” said Washington, who joined Medtronic in June 2023 after serving as VP and GM of consumer robotics at Amazon, CTO at Ford Motor Co. and VP at Lockheed Martin Space.
At Amazon, Washington said he learned that robotics isn’t just about mechatronics.

Medtronic Chief Technology and Innovation Officer Ken Washington [Photo by Hardy Wilson for Medical Design & Outsourcing]
Medtronic has a digital technology group in London designing AI technologies for surgical robotics systems, Washington said. Those features would provide surgeons with insights and guidance that could make them more efficient and effective, improving patient outcomes and reducing costs. These systems include Medtronic’s Hugo soft tissue robotics platform and the Mazor robotic guidance system for spine surgeries, though Washington declined to offer details.
“We have a strategy to continue to make our Hugo robot smarter and better and have additional instruments and end effectors as well as additional intelligence and integration with other aspects of the operating room environment,” he said. “[And the Mazor] technology is going to only get smarter and better. We have a very rich roadmap for providing additional intelligence capabilities.”
As AI technology advances, their building blocks and tools are becoming more standardized for use by designers and engineers at device developers.
“Begin with a clear identification of clinical benefits that we can provide,” Hong said. “Start from there, and then apply all those really nice tools that are ready to be used.”

Medtronic Endoscopy Chief AI Officer Ha Hong [Photo by Hardy Wilson for Medical Design & Outsourcing]
Most AI-enabled medtech products cleared, approved or authorized by the FDA are based on deep learning (which is a kind of machine learning). The simplest tools are often best when it comes to development, validation and passing regulatory scrutiny, Hong said.
“When there’s a problem, it’s much easier to find the problem and fix the issue. … If there are two technologies that achieve the same clinical benefit, you better go with a simpler model, because it has less moving parts that can go haywire.”
When to outsource AI and when to build it in-house

Dr. Salomón Zebede prepares to operate using Medtronic’s Hugo robotic-assisted surgery system at Pacifica Salud Hospital in Panama. The hospital also uses Medtronic’s AI-powered Touch Surgery Enterprise product. [Photo courtesy of Medtronic]
“Sometimes you want to be able to enter the market quickly, and so it makes sense for us to partner to accelerate our ability to get to a clinical outcome very quickly,” Washington said, citing Medtronic’s GI Genius partnership with Cosmo Pharmaceuticals subsidiary Cosmo Intelligent Medical Devices.
“AI technology — like what Cosmo had developed with the GI Genius underlying technology — we pair that with our understanding of the GI market and the GI clinical needs to enter the sales channel and deploy that product. Now we’re leveraging that to move into other therapies,” Washington said. “But in future technologies where there are additional therapies, we may choose to build that on our own. So it depends on a combination of desired speed, complexity of the technology, and when there’s a product that’s already mature enough that we could just leverage.”
When finding and vetting outsourcing partners, Washington said it’s important to take “time to get outside the walls of your own company.”
“Spend time with the broader technical community, understand how the startup community works, have a network of technology thought leaders that you can leverage for feedback,” he said. “Pressure test technologies that are presented to you to sift out what’s real versus what’s hype, to sift out what’s mature versus what’s so early-stage that it’s not a good risk to take.”
“At the end of the day, we work in a regulated industry, and we welcome that because it’s all about patient safety” Washington continued. “So if you can’t pass the test of the regulatory requirements, then it’s not going to work for us. … There are over 700 FDA-approved, AI-enabled therapies — they already have a formula for how they evaluate and approve medtech devices and therapies. We feel good about that. Our therapies and our innovations will go through that same process for approval in the U.S. and the equivalent in other regions. And so anybody looking to use AI or collaborate with a third party or a supplier should look at it through that same kind of lens.”


![A photo of the Medtronic GI Genius ColonPro polyp detection system flagging a potential sign of colon cancer during a colonoscopy. [Photo courtesy of Medtronic]](https://www.medicaldesignandoutsourcing.com/wp-content/uploads/2024/04/Medtronic-GI-Genius-doctors-268x170.jpg)

