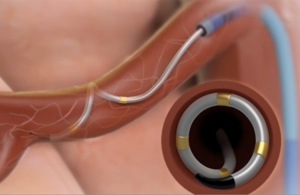
The Symplicity Spyral renal denervation system delivers energy to the nerves leading to the kidneys, which help regulate blood pressure. [Image courtesy of Medtronic]
Fridley, Minnesota-based Medtronic said last month that it hoped to present results of the Spyral HTN-ON MED trial at the Cardiovascular Research Foundation’s TCT Conference in early November, pending an interim analysis in October.
Significantly positive results would have advanced Medtronic’s efforts for FDA approval. But the independent data safety monitoring board conducting that analysis — meant to end trial enrollment if results are significantly better or worse than expected — recommended that Medtronic continue enrolling patients.
Medtronic expects follow-up of the full cohort to be complete in the second half of 2022.
The trial compares hypertension patients who undergo Medtronic’s RDN therapy after a standard renal angiography with patients who only undergo the angiography, measuring the rate of major adverse events and change in systolic blood pressure in the following six months.
Medtronic offered the latest Symplicity Spyral hypertension trial update in an Oct. 15 filing with the Securities and Exchange Commission.
“The statistically significant results from the company’s three previous sham-controlled SPYRAL studies (the ON MED and OFF MED pilot studies and the pivotal OFF MED study), and the data from the company’s Global Symplicity Registry of more than 3,000 real-world patients, give the company confidence in its renal denervation program, including ultimate approval of the therapy,” Medtronic said in the filing. “These data, in addition to the full clinical cohort, will complete the company’s robust premarket application to the U.S. FDA.”
Medtronic said it still expects a multibillion-dollar market for RDN, estimating global market revenue of more than $500 million by 2026 and $2 billion to $3 billion by 2030.
Medtronic’s fiscal year 2022 revenue and EPS guidance remains unchanged, along with its leadership’s long-range plan to deliver at least 5% organic revenue growth and 8% adjusted EPS growth.
“Management remains confident in its outlook and its pipeline, including its renal denervation program,” Medtronic said in the filing.
Analysts expect FDA approval of the Symplicity system as early as 2023.




![A photo of the Medtronic GI Genius ColonPro polyp detection system flagging a potential sign of colon cancer during a colonoscopy. [Photo courtesy of Medtronic]](https://www.medicaldesignandoutsourcing.com/wp-content/uploads/2024/04/Medtronic-GI-Genius-doctors-268x170.jpg)