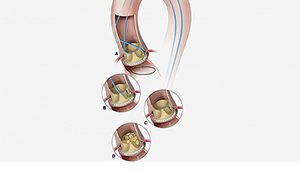
The BASILICA procedure involves (A) a catheter directing an electrified guidewire through the base of the left aortic cusp into a snare in the left ventricular outflow tract; (B) after snare retrieval, the mid-shaft of the guidewire is electrified to lacerate the leaflet (C); (D) the leaflet splays after TAVR permitting coronary flow. [Image courtesy of NIH]
The researchers at NIH’s National, Heart, Lung, and Blood Institute are calling the procedure Bioprosthetic Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction (BASILICA). They think the technique could TAVR available to even more high-risk patients who need heart valve procedures.
A leading TAVR company, Edwards Lifesciences, previously projected a $5 billion market by 2021 for the procedure. The advance at NIH could potentially accelerate TAVR’s expansion even more. About 5 million people in the United States each year receive a heart valve disease diagnosis; more than 20,000 of them die, according to American Heart Association statistics related by NIH.
A surgeon during TAVR places a catheter inside the heart, with a balloon then opening to deploy a new valve inside the aortic valve. Some patients, however, have uncommon heart structures including unusually large valve leaflets or small aortic roots. As the new valve’s scaffolding opens, the large leaflets block bloodflow to the coronary arteries.
Coronary artery obstruction only occurs during a small subset of TAVR procedures, but it is fatal more than half the time.
“There is no good treatment or prevention strategy for TAVR-induced coronary obstruction,” said Dr. Robert J. Lederman, the senior investigator in NHLBI’s Division of Intramural Research
“The previous technique of using a stent to open the coronary artery appears to have poor long-term outcomes,” Lederman said in a news release. Ledermann led NIH’s BASILICA study with Dr. Jaffar M. Khan, who developed the technique.
BASILICA involves an interventional cardiologist weaving an electrified wire the size of a sewing thread through a catheter. The wire splits the original leaflet in half. The result is that the original leaflet won’t block the coronary artery once the transcatheter heart valve has pushed it aside.
The lastest BASILICA study, published yesterday in the Journal of the American College of Cardiology: Cardiovascular Interventions, reports the successful use of BASILICA in seven gravely ill patients who qualified for compassionate use of the technique — then untested in humans.
“All patients had a successful TAVR with no coronary obstruction, stroke or any major complication,” Lederman said. “They were doing well as they reached the 30-day-mark after the procedure.”
The National Institutes of Health, the National Heart, Lung, and Blood Institute is sponsoring a multicenter early feasibility study to further evaluate BASILICA. The plan is to start enrolling patients in January 2019.




