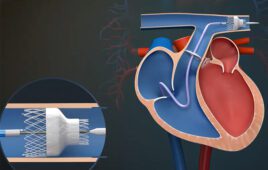
Abbott’s Amplatzer Piccolo — designed for catheter delivery inside an infant’s heart — is among the smallest pediatric devices ever made. [Image courtesy of Abbott]
Experts from Abbott, the UC Davis Pediatric Heart Center and Texas Center for Pediatric and Congenital Heart Disease tackle the challenges of designing life-saving devices for the world’s tiniest patients.
“What sets pediatric devices apart? It’s about giving children a chance at a full life,” said Dr. Lars Søndergaard, divisional VP of medical affairs and chief medical officer for Abbott’s Structural Heart division. “The solutions we design can’t just tackle the issue at hand — they need to enable normal development and stand the test of time across decades of life.”
Unlike the predictable anatomies of adults, young patients — some barely larger than the palm of a hand — are constantly changing, and those changes need to be accounted for in the long-term efficacy of the device. And though the challenges are substantial, the reward is immense. Successfully addressing an unmet need in pediatric care not only offers individual children a brighter future throughout their lives but often has cascading benefits for treating adult patients.
At DeviceTalks West 2023, a panel featuring leading physicians and device innovators discussed the unique complexities and challenges of pediatric device development. Joining Søndergaard was Dr. Byron Holt, chief of pediatric cardiology at the Texas Center for Pediatric and Congenital Heart Disease, Dr. Frank Ing, professor of pediatrics, chief and co-director of the UC Davis Pediatric Heart Center, and Katy Fraser, global marketing manager with Abbott’s Heart Failure division.
Challenge 1: Miniaturization with growth potential

Dr. Byron Holt is chief of pediatric cardiology at the Texas Center for Pediatric and Congenital Heart Disease [Photo courtesy of the Texas Center for Pediatric and Congenital Heart Disease]
“We’re making devices small enough to go through little 4 French catheters,” said Holt, highlighting the current state-of-the-art in catheter design.
However, it’s not simply miniaturization; engineers must also address how to design for patient growth. Can a valve design accommodate a heart twice its size? Will that implant stretch in proportion to the patient’s developing stature? The challenge lies in creating devices that evolve in lockstep with unpredictable yet fundamental bodily changes.
“You’re talking about a wide range of body sizes. … You have to make sure that you can get it to the size of the adult,” Ing said.
Beyond these core devices, engineers must grapple with the rest of treatment. From bulky monitoring equipment to cumbersome support systems, how can these be made more child-friendly?
“Smaller is absolutely better” and allows kids to focus on simply being kids while technology works in the background, Fraser said.
Challenge 2: Designing for durability and growth

Dr. Frank Ing is professor of pediatrics, chief and co-director of the UC Davis Pediatric Heart Center [Photo courtesy of UC Davis]
“You have to think … can we do it less invasively?” Holt said. “Their bodies are still growing. … [We] want something that can definitively address the problem without compromising normal patient growth and development.”
Adaptable devices hold the key for meeting these long-term demands.
“A successful pediatric device is dynamic, designed to evolve with the patient,” Ing said. “The challenge with many congenital issues is that you’re treating a baby when you know that rapid growth is on the horizon. Can we engineer devices that can be expanded over time, or are there biodegradable solutions that pave the way for healthier tissue to take over?”
The goal is to design for minimal interventions across a person’s lifespan, avoiding repeated surgeries whenever possible. Open, ongoing collaboration between engineers and physicians is vital. For effective solutions, engineers will benefit from an in-depth understanding of how tissues develop, the potential evolution of congenital heart defects, and the potential impact a device will have on the child’s development.
Challenge 3: Safety beyond standard precautions
Pediatric medicine demands heightened safety standards beyond simply addressing immediate complications. Engineers must meticulously consider potential impacts on brain development, lifelong radiation risks, and delicate vascular systems.
- Neurodevelopmental concerns: Repeated sedation is a concern for younger patients. “Every time we sedate a patient, we worry about neurodevelopment,” Holt said.
- Minimizing radiation risks: Pediatric patients have decades of life ahead, making cumulative radiation exposure particularly dangerous. Ing highlighted the need to find “ways to minimize radiation using non-radiation imaging systems to guide interventions.” MRI-guided technologies may be explored to address this need.
- Vascular preservation: As growing bodies may need multiple interventions, it’s vital to ensure the long-term integrity of blood vessels by avoiding obstructions or scarring that compromise later treatment access. Repeated procedures raise concerns about “losing those vessels … [so we need] ways to preserve access,” Ing said.
Challenge 4: External device demands

Abbott Heart Failure Global Marketing Manager Katy Fraser [Photo courtesy of Abbott]
“These children … move. They do things,” Fraser said, and that requires pumps, tubing, and monitoring systems that flex and adjust naturally with a child’s normal activity. Furthermore, external solutions can’t exist in a vacuum.
“You have to take into consideration lifestyle,” Fraser said. “Beyond mobility, how can equipment support a child’s education, playtime with friends, and family support — key facets of normal development?”
Design should consider intuitive use that limits burdens on parents and caretakers as well. This creates a ripple effect enhancing long-term outcomes for the child as their support system can readily engage with, maintain, and use the external devices without complex routines or specialized knowledge.
Challenge 5: Identifying the biggest opportunities

Abbott Structural Heart Division VP of Medical Affairs and Chief Medical Officer Dr. Lars Søndergaard [Photo courtesy of Abbott]
- Collaboration is crucial: Holt advocated for the breaking down of silos in favor of a shared knowledge approach from the design phase to the market. In pediatrics, the FDA has shown increased receptiveness to streamlined collaboration and early dialogue can speed up beneficial breakthroughs.
- Hybrid OR growth: Hybrid procedures blending interventional and surgical techniques will expand. Engineers developing specialized, smaller equipment and tools tailored for complex scenarios within this niche offer unique promise.
- MRI guidance: This radiation-free imaging alternative holds incredible safety advantages, but compatible tools are a limiting factor.
- The promise of bioabsorbables: Devices that serve immediate needs then disappear or self-evolve for growth remain tantalizing but difficult. A breakthrough here drastically reshapes long-term outcomes.
- Expanding the focus: Søndergaard reminds us not to overlook the mitral valve, the tricuspid valve, and the pulmonary valve. These underserved anatomical targets represent major potential growth areas for both new and improved medical devices.
DeviceTalks Boston 2024: Our favorite panels and new additions