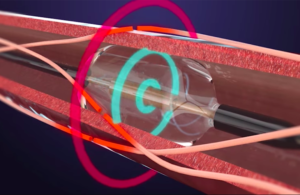
This ReCor Medical illustration shows the Paradise renal denervation catheter during the procedure. The red circle indicates ultrasound-generated energy producing heat for denervation, while the blue circles illustrate cooling from circulating water within the artery to protect the artery from heat.
ReCor Medical‘s renal denervation significantly reduced blood pressure among people with drug-resistant hypertension, according to a new study reported at the American College of Cardiology’s 70th Annual Scientific Session.
The study news, announced yesterday, could suggest a turnaround in fortunes for renal denervation technology. One considered the next big thing in the medical device industry, renal denervation took a hit in the middle of the 2010s when Medtronic announced a major clinical trial had failed to meet its efficacy endpoint.
Medtronic, though, has since sought trial designs that clear up confounding factors such as differing medication regimens and patient compliance. (Renal denervation study results involving Medtronic are expected out of EuroPCR tomorrow.) The ReCor-funded Radiance-HTN Trio study appears to have done the same, requiring all 989 people in the study upon enrollment to switch to a 3-in-1 combo blood pressure medication to better ensure adherence.
The randomized sham-controlled found a median reduction of 8.0 mmHg in daytime ambulatory systolic blood pressure after two months among those who had undergone a procedure with ReCor Medical’s Paradise ultrasound renal denervation system. The reduction was 4.5 mmHg greater than the drop experienced among those who had undergone a sham procedure.
“Radiance-HTN Trio is the first study of its kind — a sham-controlled study where all patients were placed on a guideline-recommended regimen of three antihypertensive medications and then confirmed to have hypertension resistant to this regimen,” said co-principal investigator Dr. Ajay J. Kirtane, professor of medicine at Columbia University Vagelos College of Physicians and Surgeons.
“These data confirm the blood pressure-lowering effect of the Paradise ultrasound renal denervation system in patients with resistant hypertension,” Kirtane said in a ReCor Medical news release.
Dr. Michel Azizi, co-principal investigator and professor of medicine at the Université de Paris, added that the study took place over a number of years at dozens of committed study centers in the U.S. and Europe.
“By lowering blood pressure in resistant hypertensive patients, endovascular ultrasound renal denervation may become a valuable tool in the treatment of hypertension in this broad class of patients in need,” Azizi said.
Palo Alto, Calif.–based ReCor Medical, which is owned by Japanese conglomerate Otsuka Holdings, paid for the study, with Kirtane also reporting institutional funding to Columbia University and the Cardiovascular Research Foundation.
The Paradise system bears has a CE mark in Europe and is an investigational device in the United States. It’s presently under investigation in the U.S. and Europe in the FDA IDE Radiance-HTN clinical study and in the ongoing FDA IDE pivotal study (Radiance-II), with enrollment completion expected this year.




