(Credit: Shutterstock)
The EU funded POLYACT project applied textile fabrication principles to the production of microactuators, offering a range of biomedical applications both inside and outside the body.
There are a range of advances in biotechnology which take advantage of the ability to manipulate biology at the microscopic level. Yet those fields which rely on optimum dexterity and materials compliance face significant hurdles in realising their full potential. For example, being able to grasp cells and tissues for microsurgery has typically relied on the suction power provided by micropipettes, with the risk of actually causing damage due to their rigid design and little or no force control.
Additionally, microrobotic devices driven by electric motors or pneumatic systems, are often bulky, heavy, noisy and crucially when exploited for human interfaces, feel highly artificial to the user.
The EU funded POLYACT project set out to address these limitations through the microfabrication of polymer microactuators. By exerting power in the same manner as that of muscles and motors, these actuators when patterned could then be exploited for soft, flexible micromanipulations applied to a variety of tasks. The micro-sized actuators were smaller than existing technology to one to two orders of magnitude.
Small scale production
POLYACT was able to develop, fabricate and evaluate two generations of soft and flexible microactuators, and two new fabrication methods. The team first fabricated individually controlled polyvinylidene difluoride solid polymer electrolyte (PVDF SPE) thin film based milli-actuators, but found that without a high enough ionic conductivity, the required flexibility was not achieved. The team then had to redesign the layout of the actuator and try alternatives to PVDF SPE, with interpenetrating polymer networks (IPN) proving to be the most suitable candidate. The team also had to adapt their technique of patterning highly conductive polymer electrodes, moving from a metal based approach to that which uses electronic conducting polymer layers, which were electrochemically synthesised. The resulting actuators required only a small amount of electric power as low as 20-30 mW, and low potentials (~1-2 V); allowing these micromanipulation tools, which carried their own power source, to be individually controlled.
At the beginning of the project the team highlighted the contribution that the POLYACT project offers for internal biomedicine. They cited its application in biomedical interventions such as optical shutters and diaphragms, but especially for the use of remote controlled microrobotic technology inside a patient’s body, as it reduces the risk of surgery induced trauma. However, soft manipulator technology holds out promises for a wider paradigm shift based around its characteristic of better matching the texture and consistency of biological objects, than available alternatives.
Back to the future with textuators
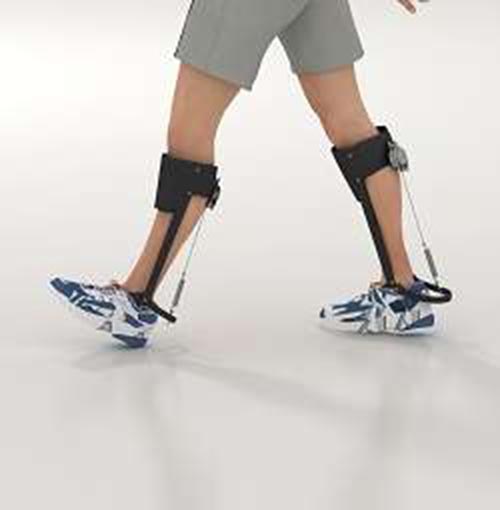
Last year members of the project team published an article in the journal Science Advances which explained how fabricating patterned actuators was a process that could be likened to textiles production and so they dubbed their creations, ‘textuators’. This paradigm allowed the team to utilise knowledge from textiles production about the relative properties – such as strength, flexibility and strain – accruing to design, depending on how it is woven and/or knitted. When combined with the feasibility of mass fabrication, the POLYACT technology holds out promise for a range of human interface applications.
The Science Advances article points to innovations in the field of assistive devices such as exoskeleton-like suits, hidden under clothes, with integrated wearable actuators that feel natural and lifelike. Here the textuators could provide muscle functions, aiding people with restricted movement such as the aged or people living with disabilities. Further down the line, the team speak of adding ‘sensing yarns into the fabric’ which would enable a feedback mechanism that would increase user control.