Many of the same factors that makes skin a fascinating, living, and breathing organism also makes it a difficult surface for most adhesives to do their job.
Sweating, shedding and constantly changing surface topography are just a few of the many challenges engineers face when choosing adhesives for their wearables, advanced dressings and other stick-to-skin medical device projects. Ignoring them will cause headaches for all involved – and maybe even a rash or two.
This is why engineers must understand key skin-related challenges – whether it’s the importance of selecting the right backing or the broad scope of factors affecting wear time – when developing solutions.

It’s important for designers to understand the full scope of factors affecting adhesion and wear time. Health, age and environment are just a few aspects to keep in mind. (Credit: 3M)
Here are six challenges engineers should account for when taking on their next project.
1. Customer requirements and communication
This may sound obvious, but we’ve heard about too many projects, particularly within the wearable devices sphere, that stumbled out of the starting blocks because design teams and their customers weren’t on the same page about adhesives. Part of that is due to the two groups speaking different languages. Engineering jargons in the adhesives world must be translated into an accessible language that everyone can comprehend. Be sure to explain requirements clearly and to keep open lines of communication. Sharing clearly defined specifications can help overcome this obstacle and reduce the product development cycle.
2. Selecting the right adhesive for the application
Every medical device adhesive isn’t meant to pair with every substrate, so choosing the right one makes for a smoother design and development process – and less irritated skin. For one, having the proper adhesive from the start can help prevent having to re-do clinical trials and studies. The right adhesive-substrate combination also means companies can avoid pitfalls like premature device fall-off, irritated skin, or difficult removal.
Here’s a brief rundown on substrates commonly used for medical devices.
- Polyester: Clear, hard, protective, and rather inflexible. However, it’s easy to adhere to and can be molded into a part, fiber, or film.
- Polyethylene: Easy to work with and conformable. Polyethylenes have a soft touch and are reasonably priced.
- Polyurethane: Ideal for wound dressing and other medical needs due to their breathability. Polyurethanes offer great flexibility and softness and can also withstand sterilization processes.
- PVC: Medical tubing is still mostly made out of PVC, which has its own series of adhesion difficulties. Although it is flexible, clear, and resistant, it is hard to dispose of and can make some tapes soft and soupy due to plasticizer migration.
- Silicone: Silicone is very popular for medical devices, yet it’s difficult to stick to, so using an alternative opens up a developer’s adhesive choices.
3. The sterilization effect
Sterilization plays an important role in killing bacteria and microbes, but the process also can negatively affect adhesive performance and a device’s functionality if not properly addressed. Take e-beam and gamma, two radiation-based sterilization methods that are among the industry’s most common. These methods can make some plastics turn yellow and cause adhesives to change properties (e.g. synthetic rubber adhesives get stickier, acrylates become firmer). The sterilization process may also make liners more difficult to release from the adhesive, and in a worst-case scenario, impossible to remove cleanly. Engineers need to understand and evaluate the effects of sterilization to avoid these issues, along with cost and time overruns in the production cycle.
4. Long-term vs. short-term wear
Skin is a dynamic organ that sheds and regenerates constantly, essentially rejuvenating every 14 days. With that, knowing how long your medical device must adhere to skin is imperative. To give engineers a more in-depth look at the wear time of available tapes, 3M recently conducted eight-day and 21-day adhesive wear time trials on human volunteers. The studies offer key findings and best practices for choosing the proper adhesive for a medical device project. Based on studies like these, and our more than half-century of experience driving innovation in medical device adhesives, here are some of the most critical questions to ask when considering long-term vs. short-term wear:
- Who is the intended end user? (e.g. neonate, children, adult, elderly)
- Is the adhesive securing a device? If so:
- How long will it be on?
- What are the device’s characteristics (e.g. material, size, etc.)?
- Where on the body will the device be secured?
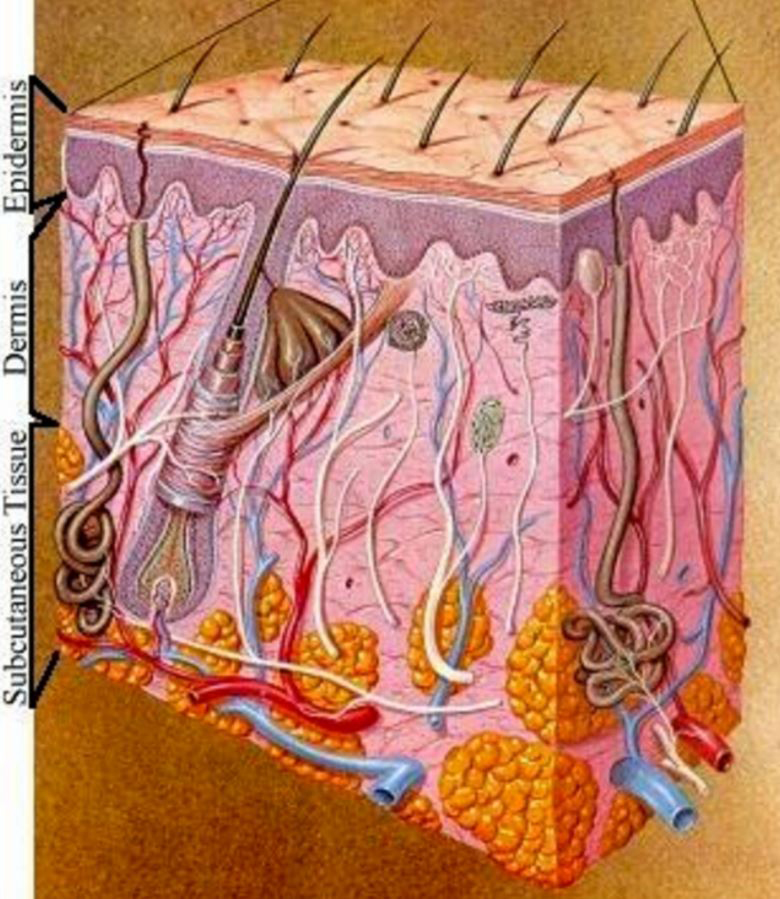
Skin sweats, sheds, constantly changes and has many layers. (Credit: 3M)
5. The importance of backing
What happens when skin feels trapped? It reacts – and not positively. In some applications, some users’ skin will eventually respond if both the backing and adhesive solution aren’t breathable. The wrong backing and adhesive combination could also damage the skin, so it’s critical that adhesives and backing are paired properly and are optimized for the application.
The right pairing impacts devices in numerous other ways. Moisture management is a key factor for long-term applications, making it important to try and use breathable adhesives and backing whenever necessary. A flexible backing is desirable, too. Some devices might require the backing to have an edge that extends beyond the device being secured, allowing the skin to move and flex around it.
6. The full scope of factors affecting adhesion and wear time
Both human and design factors come into play here. Human factors that engineers have to keep in mind include hair growth, oil, turnover of skin, and application site. It also consists of uncontrolled, human variables like culture, diet, health, environment, and age. While understanding how well and how long an adhesive sticks to the skin of an elderly person versus a baby’s may not be an engineer’s first consideration, it’s critical to know differences in skin elasticity and other characteristics because being the right fit for the end user is a device’s goal, right?
On the design side, it’s prudent to only ask an adhesive to do what the device requires. That may seem like rather elementary advice, but there can be a tendency to overdesign and use a stronger tape than necessary. If what’s being secured only needs to stay on for a couple days, designers shouldn’t pick one that’s meant to stay on for a week. Although healthy skin is durable, not all skin can handle the same level of sticking power, so using the wrong tape heightens the risk of Medical Adhesive Related Skin Injuries (MARSI). For example, some injuries are partially caused by the adhesive-to-skin bond being stronger than the skin-to-skin attachment.
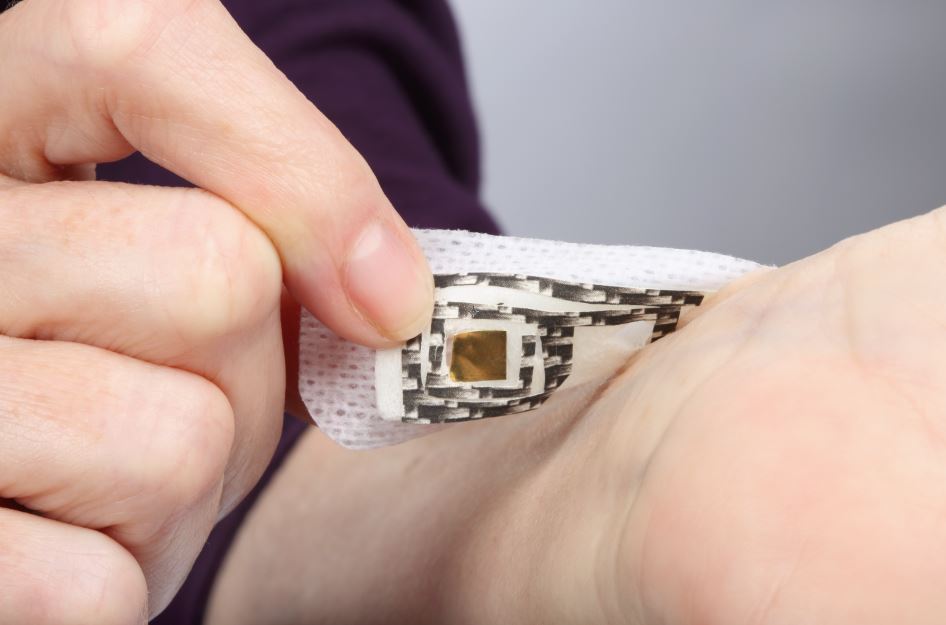
Where a device will be applied, and for how long, have big impacts on choosing the right adhesive. (Credit: 3M)
Understanding these challenges before going into projects enhances the product development process for everyone, including manufacturers, engineers and end users. Not only will there be fewer instances of irritated skin – let’s be honest, no one likes heat rash or infected hair follicles – but companies will see a more cost and time efficient process. Those who don’t address these issues can go down the wrong path from the get-go, causing their project’s timeline or budget to prematurely run out.
To avoid those negative outcomes, engineers must work with their customers to understand the complexities and features of medical tapes and how they apply to the project. They don’t have to go it alone though. Some companies, like 3M, offer support to engineers throughout the entire design and development process, providing critical analysis and discussion of adhesive options, sharing design input or helping to clarify jargon. A higher level of understanding of these stick-to-skin challenges will shine through in a project’s success.
Diana Eitzman, Ph.D., is Director of Agile Commercialization for 3M’s Critical & Chronic Care Solutions Division. Currently leading new product development and commercialization efforts in the division, Diana has over 20 years of experience at 3M in laboratory management, technology development and new product commercialization.
Kris Godbey is a Senior Applications Development Engineer in the same 3M division with over 35 years in product development and stick-to-skin technical support.




