Titan Spine announced that it has expanded the distribution of its line of endoskeleton titanium implants featuring the new nanoLOCK surface technology.
Titan Spine, a medical device surface technology company focused on developing innovative spinal interbody fusion implants, announced that it has expanded the distribution of its line of endoskeleton titanium implants featuring the company’s new proprietary nanoLOCK surface technology nationwide.
The full U.S. launch follows the successful alpha introduction of the nanoLOCK technology initiated recently in a limited number of sites. The company has achieved sales of nanoLOCK implants in 14 hospitals in 8 states since its introduction and is in the process of signing contracts with several large hospital systems.
The nanoLOCK surface technology, which is manufactured through a proprietary subtractive process, has received two differentiated government agency designations that highlight its uniqueness within the interbody fusion device market.
In late 2014, Titan Spine added an additional U.S. Food and Drug Administration (FDA) clearance for “nano-textured surface” to the product line’s initial 510(k). In October 2016, nanoLOCK was granted its own new technology category and code, formally known as an “ICD-10pcs New Technology Section X Code,” by the U.S. Centers for Medicare & Medicaid Services (CMS).
“The full launch of nanoLOCK will add to Titan Spine’s continued sales growth, highlighted by our 51% sales increase in 2015 compared to prior year,” said Steve Cichy, VP of sales for Titan Spine. “The interest in nanoLOCK has been incredibly strong and has challenged us to keep up with demand. We have generated a strong start and anticipate rapid surgeon adoption of nanoLOCK as we ramp up the full U.S. launch and showcase the technology at the upcoming North American Spine Society (NASS) Annual Meeting in Boston.”
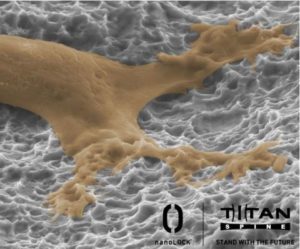
Titan Spine offers a full line of endoskeleton devices that feature Titan Spine’s proprietary nanoLOCK surface technology, consisting of a unique combination of roughened topographies at the macro, micro and nano levels (MMN).
This unique combination of surface topographies is designed to create an optimal host-bone response and actively participate in the fusion process by promoting the upregulation of osteogenic and angiogenic factors necessary for bone growth, encouraging natural production of bone morphogenetic proteins (BMPs), downregulating inflammatory factors and creating the potential for a faster and more robust fusion.
All endoskeleton devices are covered by the company’s risk share warranty.
Titan Spine
titanspine.com