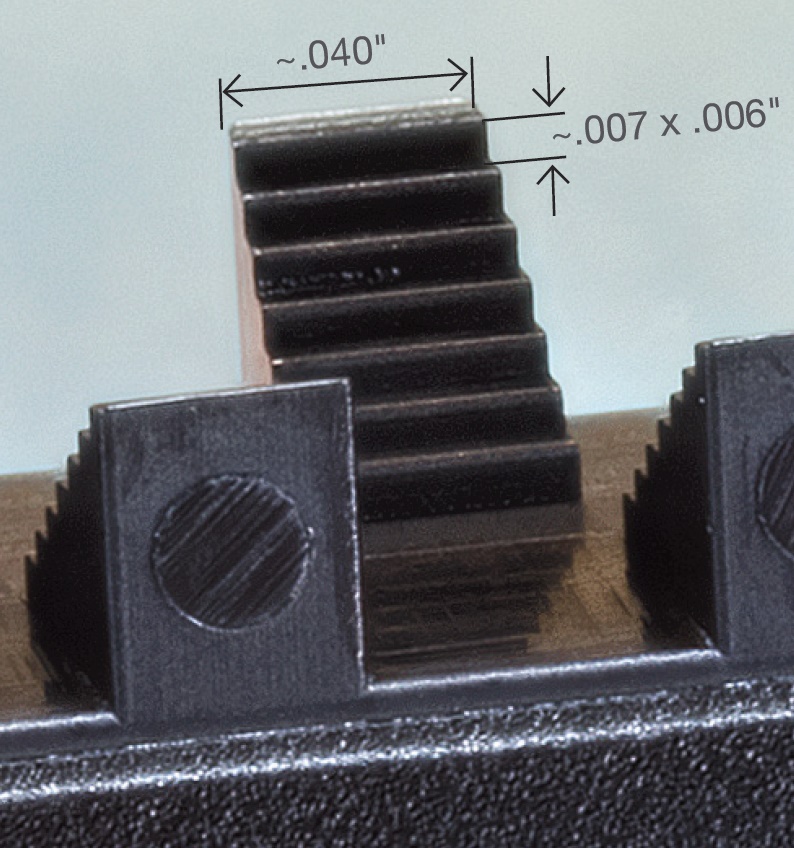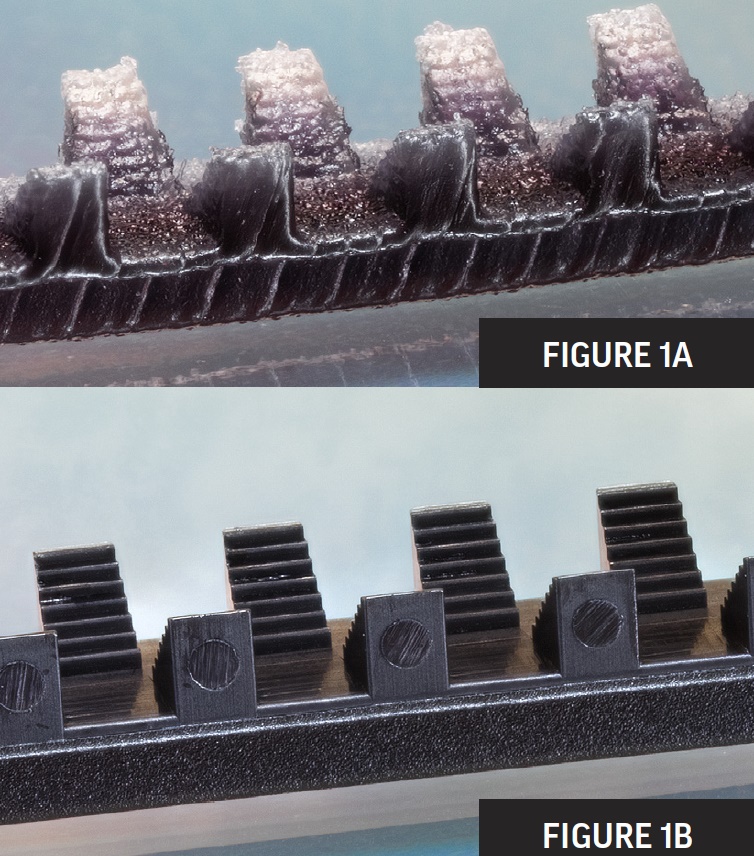
As a test of feature clarity, the top test part has been 3D printed while the bottom, micro molded. Photo: MTD Micromolding
Short lead times and design freedom have made 3D printing a trend at the macro level, evidenced by the recent FDA clearance of the Unite3D foot & ankle fixation device by Zimmer Biomet, made with the company’s proprietary OsseoTi porous metal technology.
But 3D printing’s advantages may not translate on a micro-scale product. Because it’s a 100% additive process, meaning that it converts engineering data directly into a three-dimensional product, no forming tools are used to shape the finished product. Meeting location- and tolerance-based requirements depends on the accuracy and repeatability of the 3D printing process and material. That’s a tall order at the micro scale, where a large dimension is 0.05 in., as shown in the accompanying photos.
The answer to whether 3D-Printing will be sufficient or whether a part must be hard-tooled in a micromold might become clear after determining the need for micro-sized features. Ask these few questions, suggests the engineers at MTD Micro Molding: Are the details required or “critical-to-function”? The same is true for the number of parts required: Is the need limited to a handful of parts, or thousands? The suitability of materials is also crucial, especially when the design requires dynamic, dielectric, biocompatibility, or rate of absorption testing.
The top image (in the stacked pair) is a 3D-printed prototype made using a high-precision printer capable of deposition layers at 16-µ thick and 25-µ offset. The bottom image shows the comparison micro-molded part, made using a combination of several processes, including injection molding, tooling, and material-science evaluation.