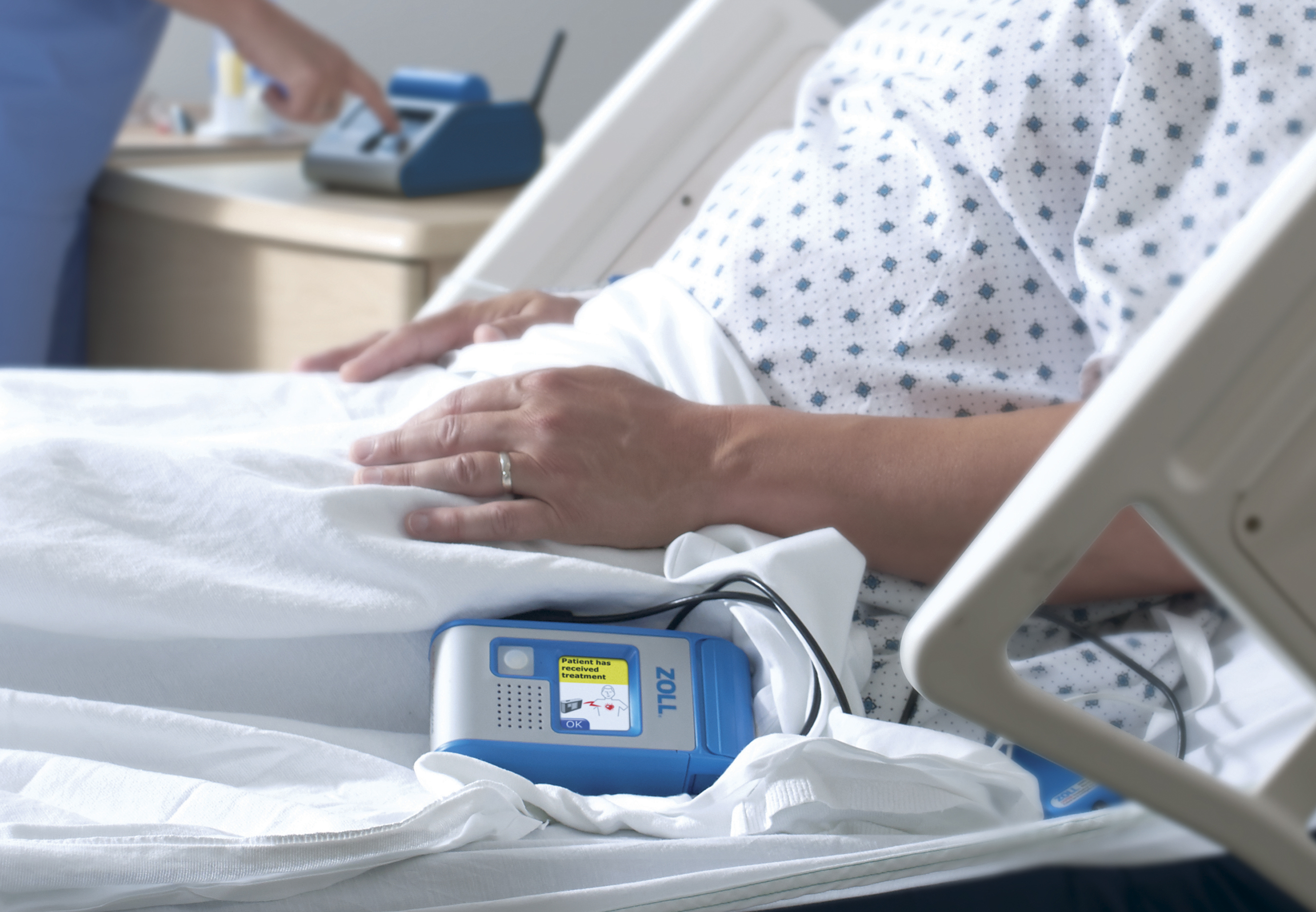
The ZOLL Hospital Wearable Defibrillator approved by FDA (Credit: Business Wire)
ZOLL Medical Corporation, an Asahi Kasei Group Company that manufactures medical devices and related software solutions, announced today that the company’s Hospital Wearable Defibrillator (HWD) has been granted premarket approval (PMA) by the U.S. Food and Drug Administration (FDA) to market and begin U.S. distribution of the device.
The ZOLL HWD is specifically designed to continuously protect patients at risk of ventricular tachycardia or ventricular fibrillation (VT/VF) during their stay in the hospital. For a patient experiencing VT/VF, each minute that defibrillation is delayed increases the likelihood of dying or the likelihood of surviving with complications.
Through automatic detection and immediate treatment, the ZOLL HWD provides patients at risk for VT/VF with continuous protection anytime day or night in the hospital. The HWD is an innovative tool for hospital care teams to manage patients at risk of VT/VF. Automatic detection and immediate treatment address the most critical factor for survival from cardiac arrest in these patients—timely defibrillation.
“The HWD offers hospital care teams a new option for managing patients at risk of VT/VF by providing continuous protection even outside of very expensive, high-acuity care areas,” says Jonathan A. Rennert, CEO of ZOLL. “It complements the tool kit the care team has in place for responding to cardiac arrest.”
“The ability to provide hospitalized patients at risk for malignant ventricular arrhythmias a safe and effective device that enables rapid defibrillation represents a significant improvement in the care of cardiovascular patients,” says David M. Shavelle, MD, Associate Clinical Professor at Keck School of Medicine Division of Cardiology at the University of Southern California (USC), Director of Los Angeles County (LAC) USC Cardiac Catheterization Laboratory, and Director of General Cardiology Fellowship Program at USC.
Automatic Detection, Immediate Treatment
The HWD helps the care team manage at-risk patients by detecting VT and VF. If a life-threatening rhythm is detected, the device alerts the patient prior to delivering a treatment shock, and thus allows a conscious patient to delay the treatment shock. The device is designed to deliver treatment within 60 seconds.
The HWD utilizes the same detection algorithm and defibrillation waveform as the LifeVest Wearable Defibrillator. Patients wearing the LifeVest have a 98 percent first shock success rate and 92 percent event survival rate.




