Pixium Vision announced that it has been awarded CE mark for its IRIS II bionic vision system. This 150-electrode epi-retinal implant features a design intended to be explantable and upgradeable. The IRIS II system is now CE mark approved for people with vision loss from outer retinal degeneration.
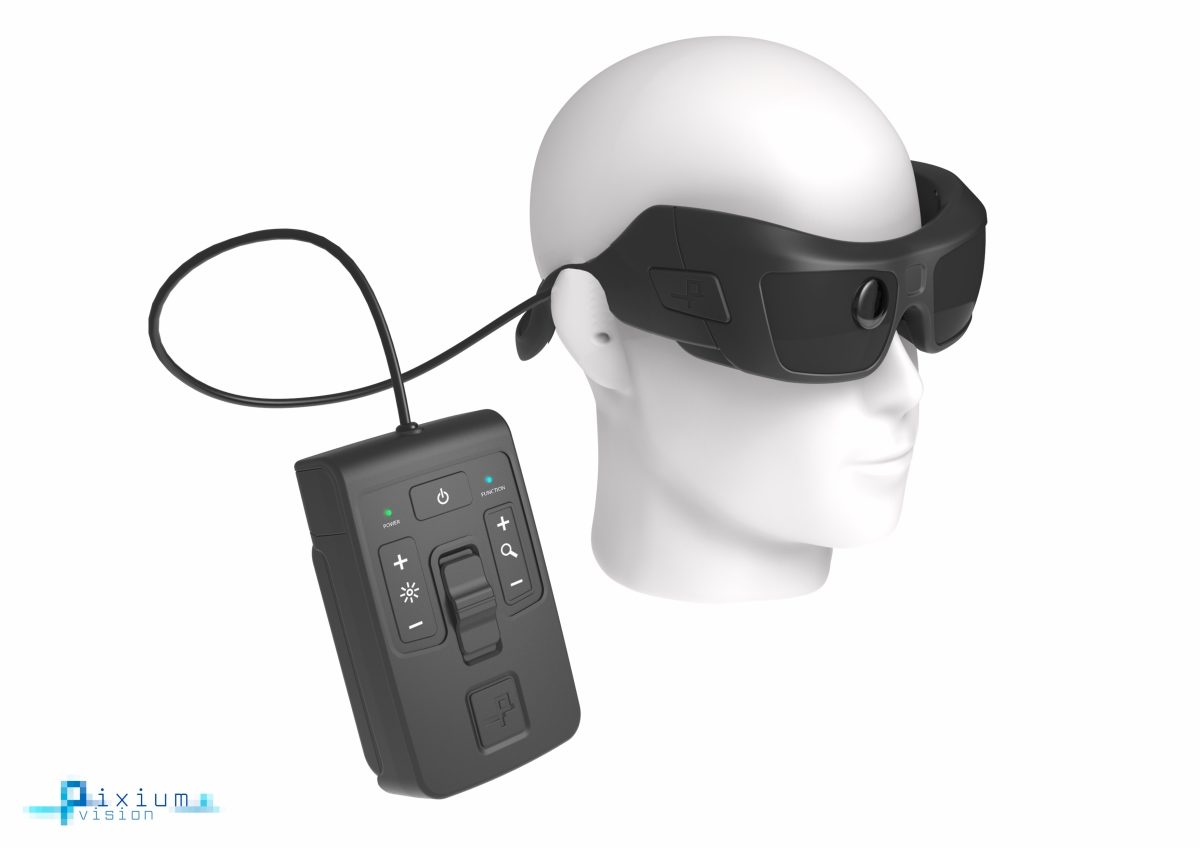
Photo Credit: Pixium
IRIS II incorporates innovative and distinctive features:
– A bio-inspired camera intended to mimic the functioning of the human eye by continuously capturing the changes in a visual scene with its time independent pixels, and unlike an imaging sensor that takes a sequence of video frames with largely redundant information;
– An epi-retinal implant with 150 electrodes – almost three times the number of electrodes than previous version;
– An explantable design: the electrode array is secured on the retinal surface by a patented support system that is intended to allow for explantation or future replacements or upgrades.
The IRIS II system is only available by medical prescription. Several leading ophthalmology centers in Europe are continuing to evaluate the system’s long-term performance based on a pre-defined protocol. The company is now able to file for national reimbursements.
Khalid Ishaque, CEO of Pixium Vision said: “The CE mark certification is a major step forward for Pixium Vision and for retinal dystrophy patients who have lost their sight. This recognition, by an independent expert body, validates the long-term multidisciplinary work that has resulted in market approval of the IRIS II system. We will continue to develop our bionic vision systems with the aim to deliver improved visual perception and help retinal dystrophy patients lead more independent lives.”
In parallel, Pixium Vision is developing a tiny, wireless, sub-retinal photovoltaic implant for patients with AMD (age-related macular degeneration).




